Joint press release by Hokkaido University and University of Hyogo
New research employs shutter speed analogies to validate 55-year-old theory about chemical reaction rates.
As the observation interval lengthens-akin to slowing down a camera's shutter speed-the dances of the molecules overlap and emerge as a blur of frequent changes, masking the intricate ballet of atoms in motion. (Illustration: Yumi Teruya)
Chemical reactions are commonly depicted as transitions from reactants to products. However, such reactions involve many molecules, and the individual molecules themselves undergo frequently-occurring structural changes as they transform from the reactants to the products. Even in the most straightforward chemical reactions, the actual observable changes that occur during the reaction are far quicker and far more complex than can be observed with any existing technology―similar to how fast-moving objects appear blurry in photos taken with a slow shutter speed.
A research team in Japan, led by Professor Tamiki Komatsuzaki at the Institute for Chemical Reaction Design and Discovery (ICReDD), Hokkaido University, have developed a framework that accurately describes how first-order reactions appear depending on the time interval used to measure the reaction. Their work was described in the journal Proceedings of the National Academy of Sciences.
"During a reaction, the atoms of the reactants and products undergo a series of structural rearrangements, or isomerization, until the reaction is complete" Tamiki explains. "The speed at which these isomerizations occur means that we typically obtain only a simplified understanding of the process at any given point, through a process called coarse-graining."
Yutaka Nagahata, first author of the study, elaborates, "We formulated a coarse-graining process that satisfies 'exact lumping' conditions-the exact matching of a simplified version of an equation with its original detailed counterpart, a theory proposed over half a century ago-by focusing on observation intervals, which can be thought of as the 'shutter speed' of a scientific observation. To adopt this matching, we formulated a criterion for the indistinguishability of the statistical behavior of stable molecular shapes (isomers) as a function of observation 'shutter speed'."
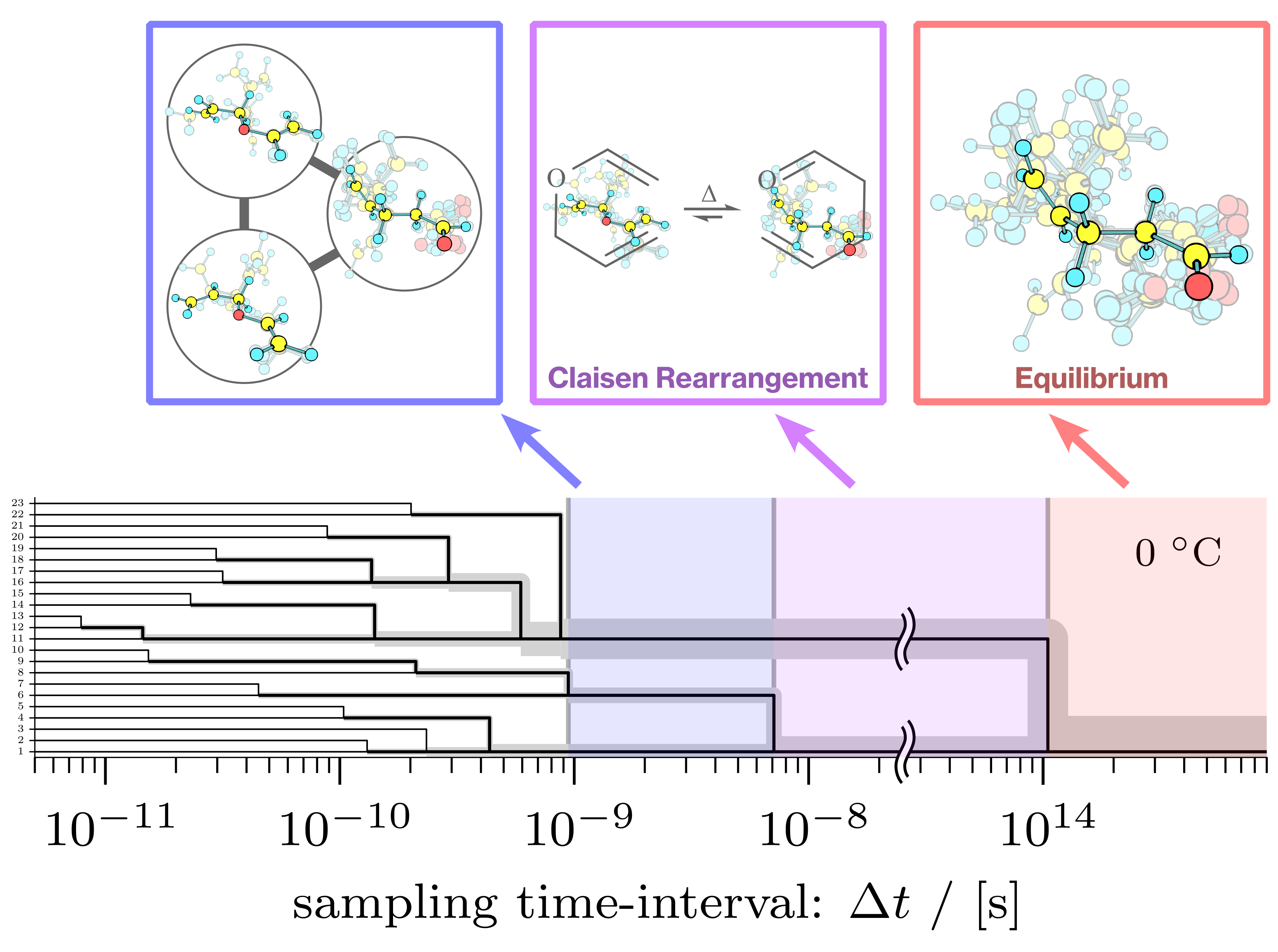
The dendrogram of indistinguishability of the Claisen rearrangement of allyl vinyl ether. Each colored step indicates an observation where exact coarse-graining applies. (Illustration: Yutaka Nagahata)
The team identified key observation intervals at which different molecular shapes "blur together" and the system appears to become simpler. They created a "systematic diagram" that shows how the reaction process appears more and more simplified as the observation interval increases, eventually appearing a one-step process (reactants directly changing into products) at long observation intervals. Using this "systematic diagram," it is possible to immediately determine indistinguishable groups and obtain the rate equation over the groups by applying developed exact lumping.
"In developing exact coarse-graining, we have leapfrogged over current approximation theories, which possess many omissions that make them quite inaccurate," says Professor Mikito Toda at the Graduate School of Information Science, University of Hyogo, and a coathour of the study.
The team used the Claisen rearrangement reaction of allyl vinyl ether to show that exact coarse-graining could account for all possible reaction paths. Future work will focus on extending this study to other first-order reactions. Ultimately, the researchers hope their work will provide a mathematical support of transition state theory.

(From left) Yutaka Nagahata, Masato Kobayashi, Mikito Toda (University of Hyogo), Satoshi Maeda, Tetsuya Taketsugu, and Tamiki Komatsuzaki of the research team. (Photos: WPI-ICReDD; Mikito Toda)
Original Article:
Yutaka Nagahata, et al. An encompassed representation of timescale hierarchies in first-order reaction network. Proceedings of the National Academy of Sciences. May 17, 2024.
DOI: 10.1073/pnas.2317781121
Funding:
This work was partially supported by the Japan Society for the Promotion of Science (JSPS) through the Cooperative Research Program of "Network Joint Research Center for Materials and Devices" (20221095), Grants-in-Aid for Scientific Research (B) (23H01915, 17H02940), Grants-in-Aid for Scientific Research (C) (19K03653, 23K03265), Grant-in-Aid for challenging Exploratory Research (16K14703); and a Grant-in-Aid for Scientific Research on Innovative Areas (18H05408) from the Ministry of Education, Culture, Sports, Science and Technology of Japan (MEXT).






