EMBL researchers have found a new way to grow and monitor very early embryos in the laboratory, opening up a rare window into a poorly studied stage of development
One of the most common causes of infertility is failure of implantation - the process by which the early embryo attaches to the wall of the uterus. However, despite its critical importance, implantation and the changes it induces in an embryo are notoriously difficult to study.
A recent study by EMBL researchers proposes a new method to grow early embryos in the laboratory. Using a 3D culture setup, scientists can closely monitor embryo changes around the time of implantation. The study results from a collaboration between teams led by former EMBL group leader Takashi Hiiragi (currently at Hubrecht Institute, KNAW, the Netherlands), and present EMBL group leaders Anna Erzberger and Anna Kreshuk.
"Implantation is a crucial step of development for mammals," said Takafumi Ichikawa, one of the first authors of the study published in Developmental Cell. "However, with methods that previously existed in the field, studying peri-implantation development in the embryo has been challenging."
Many of these earlier methods grew the embryos on flat surfaces in two-dimensional cultures. The researchers hypothesized that this might cause embryo cells to spread out abnormally and disrupt the process of morphogenesis - tissue formation. To avoid this, they decided to come up with a 3D culture system that more closely mimics in vivo conditions.
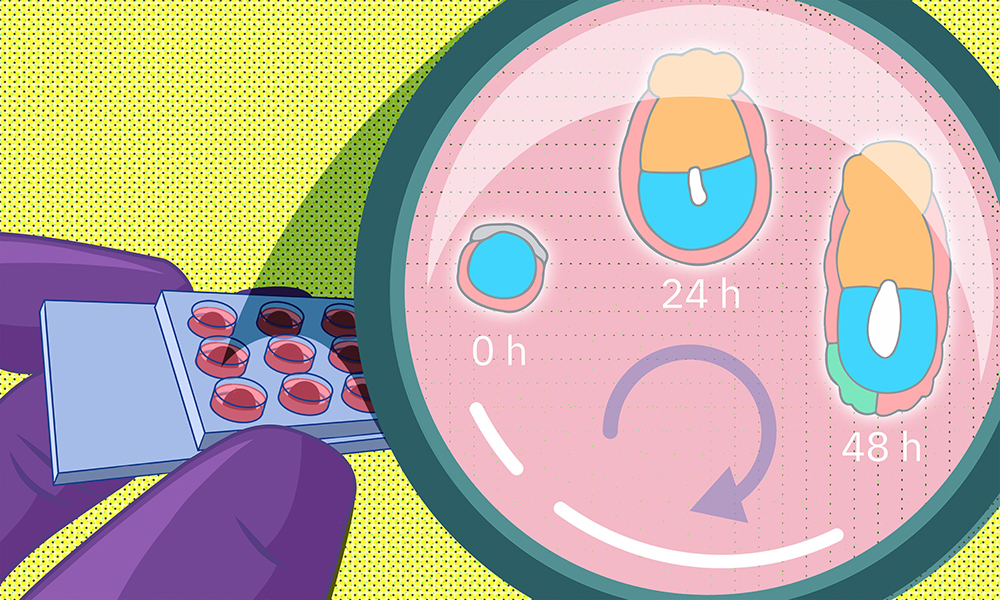
To do this, the researchers collected mouse embryos four and a half days after fertilisation and embedded them in a 3D gel matrix. Within this matrix, the embryos could grow for up to 48 hours, with a developmental trajectory that closely mirrors what happens in vivo. Through a collaboration with Lars Hufnagel, a former EMBL group leader and the Kreshuk group, the researchers also integrated the culture system with state-of-the-art light-sheet microscopy and machine-learning-based image analysis protocols. This allowed them to follow the embryo development in real time and perform detailed quantitative analyses not possible before.
"Conventional manual image analysis entails tremendously time-consuming and labour-intensive work," said Hui Ting Zhang, a PhD student in Hiiragi group and the paper's co-first author. "Our method allows us to process a much larger volume of data without compromising the resolution."
Through their collaboration with the Erzberger group, the researchers used such data to reveal critical insights about the biophysical aspects of development. In particular, this model allowed the researchers to investigate closely the interactions between tissue layers that give rise to the embryo proper and tissue layers that lie outside the embryo and help support its growth. Mechanical interactions between such embryonic and extra-embryonic tissues turned out to be critical for a lumen (a fluid-filled cavity) to form within the embryo, which eventually gives rise to the amniotic sac.
"The system allowed us to investigate the importance of a properly shaped interface between embryonic and extra-embryonic tissues for lumen formation," Erzberger said. "The fact that live imaging is possible with the ex vivo system meant that we could look at the dynamics of lumen formation."
The researchers plan to continue improving their model and apply it to investigating early developmental pathways in mammalian embryos. "The better our understanding of the implantation process is, the more insights we will be able to apply in the biomedical context, ultimately to solve pertinent issues such as infertility," said Zhang.






