UC San Francisco has launched the Baszucki Lymphoma Therapeutics Initiative to increase the effectiveness and availability of chimeric antigen receptor T-cell (CAR T) therapy for lymphoma patients. Jan Ellison Baszucki and her husband, Roblox CEO David Baszucki, gifted $6 million over five years to support the initiative. After witnessing Jan's father recover from a near-fatal lymphoma thanks to treatment with CAR T therapy, the couple wanted to help accelerate progress in the field so more families can benefit.

An emerging approach to immunotherapy, CAR T therapy has dramatically increased survival rates for patients with certain hard-to-treat blood cancers. It also shows promise for treating some solid tumors as well as diseases beyond cancer, including autoimmunity and neurodegeneration. The therapy involves harvesting T cells from a patient's blood and genetically engineering them to kill cancer cells by detecting specific molecular targets, called antigens, on the cancer cells' surface. The engineered T cells are then cultured to increase their numbers and infused back into the patient's bloodstream, where they act as a "living therapeutic" against the cancer cells.
"In cancer, we don't often use the big C word - cure - but CAR T therapy really does seem to lead to 'miracle cures' after no other therapies have worked," says Babis Andreadis, MD, MSCE, a lymphoma specialist and professor of clinical medicine at UCSF who will direct the new initiative.
Since 2017, the US Food and Drug Administration (FDA) has approved five CAR T therapies for acute lymphoblastic leukemia (ALL), multiple myeloma, and several lymphomas. Andreadis ran some of the first clinical trials of CAR T therapies for non-Hodgkins lymphoma, which have shown complete remission rates of around 50 percent in patients who have failed multiple prior treatments.
In cancer, we don't often use the big C word - cure - but CAR-T therapy really does seem to lead to 'miracle cures' after no other therapies have worked.
The Baszucki Lymphoma Therapeutics Initiative will expand research underway at UCSF aimed at understanding who will benefit from CAR T therapy and developing new therapeutic targets for lymphoma patients who don't respond to current CAR T therapies.
"Our goal is to reach a point when cancer medicine is completely personalized and every patient can get a therapy designed specifically for their own disease," says Alan Ashworth, PhD, FRS, president of the UCSF Helen Diller Family Comprehensive Cancer Center and the E. Dixon Heise Distinguished Professor of Oncology. "Jan and David's commitment to helping realize this vision exemplifies their remarkable foresight and generosity."
'Big, Bold Moves'
The Baszuckis, who live in San Francisco with their four children, are veterans of the Bay Area technology industry. Jan was the director of marketing at Infinity Financial Technology before becoming an award-winning writer and novelist. David is a software engineer and co-founder of Roblox Corporation, which develops Roblox, an online gaming platform that allows users to create and play their own games.
After the company went public, in March 2021, the couple launched the Baszucki Group to create foundational change through philanthropy, impact investing, community building, and other initiatives. One of the organization's first projects was establishing the Baszucki Brain Research Fund, which supports scientists and innovators pursuing breakthrough treatments that promote vitality, stability, and well-being in people at risk for or living with bipolar disorder. This year, the fund has invested more than $13 million in research grants, including a competitive program that will fund 45 grants to support pilot research in therapeutic discovery and translational research for bipolar disorder.
"Our goal with Baszucki Group is to direct resources toward the greater good," Jan Baszucki says. "We're interested in big, bold moves that will leapfrog current treatments rather than make incremental progress. It's obvious that CAR T therapy fits into that category."

"One of the exciting things about CAR T is it's not just one product," David Baszucki adds. "It's a revolutionary therapeutic platform that offers infinite avenues for innovation, and Dr. Andreadis is one of those brilliant researchers who can use it to make CAR T therapy a reality for more families like ours."
The new initiative will also further the goals of the UCSF Living Therapeutics Initiative (LTI), which launched in June. By uniting many established UCSF programs, the LTI seeks to propel research in living therapeutics - a broad category of medicines that use human or microbial cells to treat disease - and speed promising therapies to clinical trials for patients who lack effective treatment options.
"UCSF is laying the groundwork for the next frontier in pharmaceutical medicine," says Sam Hawgood, MBBS, UCSF chancellor and the Arthur and Toni Rembe Rock Distinguished Professor. "We are deeply grateful to the Baszucki family and other philanthropic partners who inspire everyone engaged in our mission to treat patients compassionately and equitably with the best therapies they can get anywhere in the world."
New Hope for 'Untreatable' Cancers
The Baszuckis first met Andreadis in October 2020, after Jan's father, Todd Ellison - also an award-winning novelist, who writes under the pen name E.T. Ellison - had endured three years of chemotherapy while only growing sicker. "I was told I had no options left," Ellison says. That's when his oncologist, who had trained at UCSF, referred him to Andreadis.
Earlier that fall, Andreadis had launched a study to test the viability of administering CAR T therapies manufactured in a university setting. Clinical researchers have previously partnered with pharmaceutical companies to use CAR T products already on the market or in development for commercialization. These products typically are manufactured by research contractors or by the companies themselves because they require specialized clean rooms, called current good manufacturing practice (cGMP) facilities, to ensure that the drugs are safe and of the highest quality.
However, some universities recently have begun making CAR T and other cell-based therapies in house. In June of this year, as part of the LTI, UCSF announced a strategic alliance with Thermo Fisher Scientific Inc. to build and manage a cGMP facility in leased space on UCSF's Mission Bay campus. The new facility will allow clinicians to offer cell therapies to more patients, including those who may be ineligible for or can't afford commercial products. In-house manufacturing also will allow researchers to optimize production specifications and doses and develop new therapeutic targets that drug companies might not pursue.
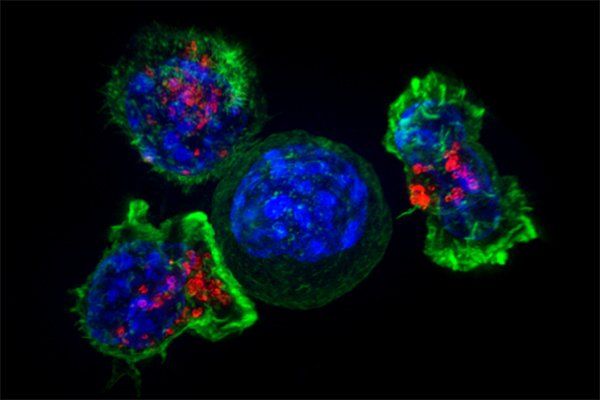
"The CAR T products available today are designed for generic targets," Andreadis says, adding that while this approach maximizes the number of patients who are likely to respond to a particular CAR T product, it also means that patients whose cancers express low levels of these targets might not benefit.
Andreadis's study is a phase I trial to test the safety and appropriate dose of CAR T cells manufactured under custom protocols to target a common FDA-approved antigen known as CD19. (Until the cGMP facility at UCSF is operational, Andreadis's team is making the cells at a facility at UC Davis.) Ellison was the first patient to enroll. "When he arrived here, he was seriously ill," Andreadis says.
Within a week of starting the treatment, however, Ellison began to improve. "We kept waiting for him to get worse, but he just got better and better," Jan Baszucki recalls. "And there were zero side effects, none at all," Ellison adds. By December 2021, his lymphoma was gone, and he remains cancer-free. (Not all CAR T patients have the same experience; some patients may experience short- or long-term side effects, including cytokine release syndrome and neurotoxicity.)
"We are beyond grateful to Dr. Andreadis and his staff for the incredible care given to my father," Jan Baszucki says.
Andreadis's team has since treated five more patients in the phase I trial, several of whom had lymphomas that couldn't be treated with commercial CAR T products or who would not have qualified for insurance coverage because they were too sick. With the Baszuckis' support, the team can now expand the study to enroll more patients with various lymphomas, including rare subtypes. They also plan to use patients' blood samples to begin investigating new disease targets for the next generation of CAR T therapies.
Beyond improving treatment options for lymphoma patients, Andreadis predicts, this pioneering work will help set the stage for the development of in-house CAR T products for other cancers and diseases. "Clinicians and scientists around the world will be able to use the infrastructure we are creating here at UCSF to customize CAR T therapies for a broad range of applications," he says.






