Hepatocellular carcinoma (HCC) is two to five times more common in men than in women. An in-depth analysis of the mechanisms underlying the gender heterogeneity of HCC may contribute to the identification of key HCC suppression mechanisms physiologically present in women, thus benefiting the HCC cohort.
A cytochrome P450 family member with female-preferential expression blocking hepatocarcinogenesis
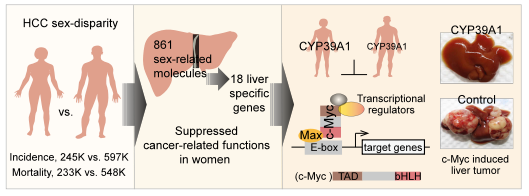
Against this backdrop, Prof. JI Junfang at the Zhejiang University Life Sciences Institute led her team to delve deeply into multiple omics data which revealed 861 gender-related molecules in non-tumor liver tissues between female and male HCC patients. The expression patterns of these molecules in female livers were intimately bound up with the suppression of cancer-related diseases and functions. These findings were published in the journal Gut on January 7, laying a key molecular biological basis for the significantly lower incidence of HCC in women than in men.
A member of cytochrome P450 family, CYP39A1, was one of the top liver-specific candidates with significantly higher levels in female vs male livers. In HCC tumors, CYP39A1 expression was dramatically reduced in over 90% HCC patients. Exogenous CYP39A1 significantly blocked tumor formation in both female and male mice and partially reduced the sex disparity of hepatocarcinogenesis. The HCC suppressor role of CYP39A1 did not rely on its known P450 enzyme activity but its C-terminal region, by which CYP39A1 impeded the transcriptional activation activity of c-Myc, leading to the remarkable inhibition of hepatocarcinogenesis.
"The research reveals that the liver-specific CYP39A1 with female-preferential expression was a strong suppressor of HCC development," said Prof. Ji. "Regulating CYP39A1 might be a promising strategy for HCC treatment in both women and men patients in the future."






