The team led by Prof. CHEN Shuqing and Prof. PAN Liqiang at the Zhejiang University College of Pharmaceutical Sciences published their latest research findings regarding a multivalent biparatopic EGFR-targeting nanobody drug conjugate in the journal Signal Transduction and Targeted Therapy on September 3.
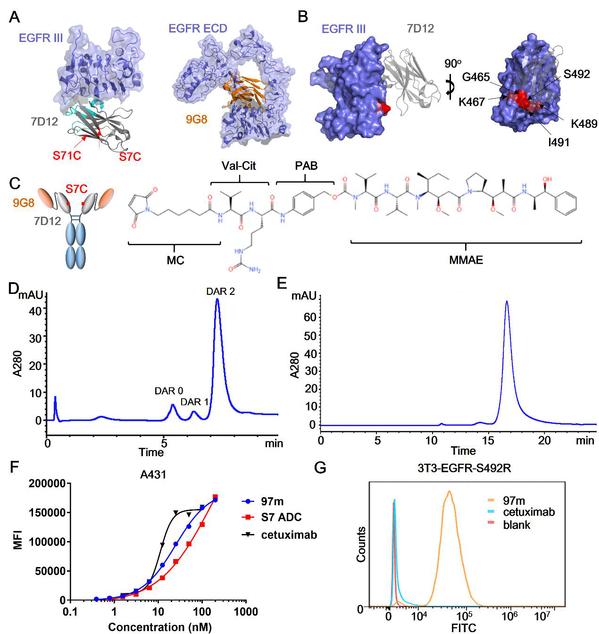
Design of multivalent anti-EGFR nanobody drug conjugate
An antibody-drug conjugate (ADC) is a type of anticancer drug linking an antibody with cytotoxic molecules. It integrates the targeting properties of the antibody and the killing effect of the chemotherapeutic drug. EGFR-targeted ADCs can specifically deliver cytotoxic molecules to the cytoplasm of EGFR-positive tumor cells by targeting antigens on the cell surface and then internalizing them into intracellular lysosomes for degradation, thereby overcoming the drug resistance of some targeted drugs induced by mutations in EGFR downstream signaling pathways. Prof. CHEN Shuqing et al. constructed a tetravalent biparatopic anti-EGFR ADC, which consists of two fused anti-EGFR nanobodies targeting two distinct non-overlapping epitopes. This drug proved to exhibit superior endocytosis than cetuximab and play a better role in delivering toxins into tumor cells. It could also evade targeting resistance hotspot mutation to cetuximab or panitumumab, therefore having promising therapeutic efficacy for the treatment of EGFR-overexpressing solid tumors with or without acquired point mutations.
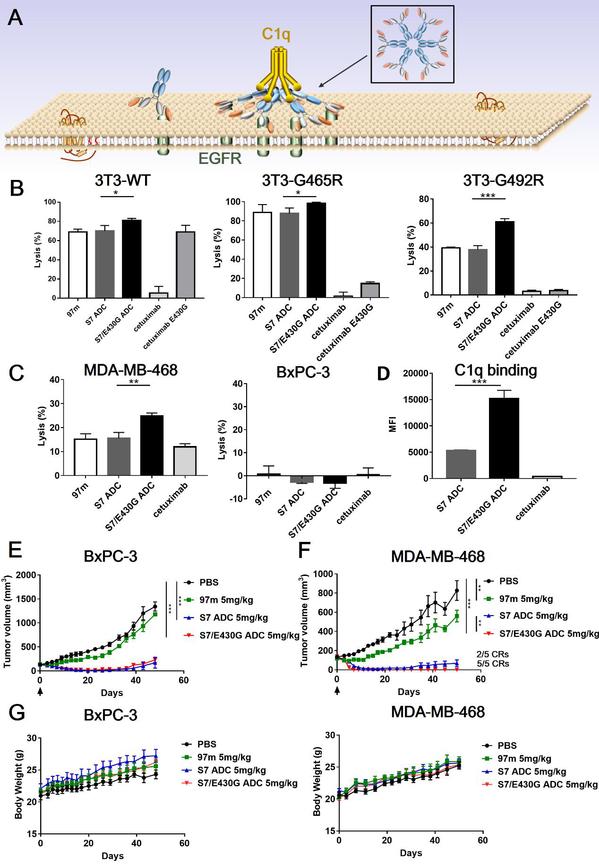
In vivo antitumor efficacy of the antibody-drug conjugate
In addition, while confirming that ADCs could activate complement-dependent cytotocity (CDC) more effectively than conventional anti-EGFR antibodies, researchers also attempted to modify the Fc domain to promote the CDC effect and develop a CDC-enhanced ADC. They explored the possibility of applying CDC enhancement to ADC drugs and found that this CDC-enhanced EGFR-targeted ADC displayed better anticancer activity, thus broadening the scope of ADC design.
"This novel ADC could significantly enhance targeted anticancer activity as a "biological missile" and help address common mutation resistance in EGFR-targeted therapies," said Prof. Chen. "It is expected to offer more options for those patients with malignant tumor."






