Pancreatic cancer is difficult to detect, in part because the pancreas sits deep in the abdominal cavity in a position that can vary from person to person; pancreatic tumors therefore can remain hidden until too late for treatment. Now, researchers at the Weizmann Institute of Science have demonstrated how an emerging magnetic resonance imaging (MRI) approach could make pancreatic tumors "light up" in MRI scans.
Similar to glucose tolerance tests, which can indicate the onset of diabetes by measuring how the body digests sugar, the new MRI method tracks how cells "eat" - that is, metabolize - glucose.
Nearly a century ago, German Jewish scientist and Nobel Prize laureate Otto Warburg discovered that tumors consume unusually large amounts of glucose relative to most non-cancerous cells. He also noticed that most glucose consumed by tumors ferments to lactate, a phenomenon that became known as the Warburg effect. By showing how MRI can be used to distinguish and map the specific metabolic products that, as a result of the Warburg effect, arise only in cancer cells, the new Weizmann MRI method might offer a way to "check in" and identify the presence of pancreatic cancer. This method could lead to earlier detection, better treatment and a more hopeful outcome for pancreatic cancer patients.

A new chemistry for tracking glucose metabolism
The research, conducted using rodent models of aggressive pancreatic cancer, was performed in Prof. Lucio Frydman's lab in Weizmann's Chemical and Biological Physics Department in collaboration with Prof. Avigdor Scherz of the Plant and Environmental Sciences Department. To develop the novel MRI method, the scientists used a chemically altered glucose containing a stable isotope of hydrogen called deuterium. Prior to scanning, this altered glucose was injected into the bloodstream of mice with pancreatic cancers.
According to Frydman, the method may outperform traditional MRI or positron emission tomography (PET) scanning techniques, both of which have a poor record of identifying pancreatic tumors.
"Traditional MRI fails to detect pancreatic tumors because, even when external contrast agents are added, the scanning is not specific enough to highlight the presence and location of the cancer," says Frydman. "Doctors can't see the tumor until the patient feels its effects. Even when the scan indicates an abnormality, it often cannot be distinguished from an inflammation or a benign cyst. Likewise, PET scans cannot necessarily be trusted because a positive scan does not always mean the patient has cancer, and a negative PET scan does not always mean the patient is cancer free," he explains. He adds that standard preventive care for pancreatic cancer involves periodic CT and MRI scans, often accompanied by invasive and uncomfortable endoscopic biopsies, but this combined approach rarely works.

Seeking to fill the gap left by this paucity of diagnostic methods, Frydman and his team set out to discover new signatures of pancreatic cancer by using MRI to map the different ways in which normal and cancerous tissues metabolize glucose.
"We all love sugar - who can say no to a good ice cream sundae!" Frydman says. "In healthy cells, the end product of glucose digestion is CO2, the gas we exhale when we breathe out. But Otto Warburg discovered that cancer cells do not eat glucose 'all the way.' Instead, glucose digestion stops at an intermediate point to produce lactate, a molecule that is believed to play an important role in cancer cell division and proliferation."
""In conventional MRI, the water signal is simply blinding, and the lactate - the calling card of cancer cells - goes undetected"
Frydman explains that stopping at this midpoint of glucose digestion causes cancer cells to produce less energy than normal cells. However, this "eating style" gives the cancer cells a survival advantage: The presence of lactate greatly helps cancer cells to multiply and destroy surrounding tissues. "Our goal was to use this fact, together with MRI, to reveal the specific locations where lactate is produced, thereby identifying the presence and location of cancer cells and tumors."
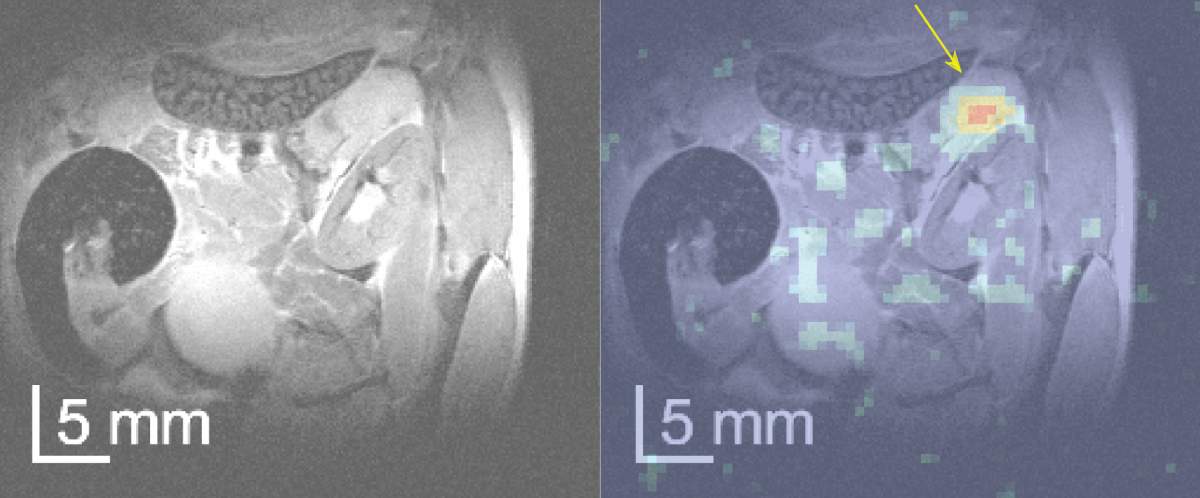
"Work-arounds" to increase MRI sensitivity
But there was a problem: The amount of lactate produced in the cancer cells was far below the detection threshold of conventional MRI, which works by measuring the abundant protons in the water contained in tissues being scanned. To locate the lactate, the scanning technology must overcome the huge signal created by the water itself, which has a proton concentration about 100,000 times greater than the lactate that the Warburg effect would produce. "In conventional MRI, the water signal is simply blinding, and the lactate - the calling card of cancer cells - goes undetected," Frydman says.
To overcome this, he and his team introduced two work-arounds. First, they exchanged glucose's protons with deuteriums, which are a nonradioactive form of hydrogen. When this "deuterized" glucose was eaten by the cancer cells, it produced deuterized lactate, which came significantly closer to producing a readable MRI signal, as it was no longer drowned out by the signal of the water-borne protons. These lactate signals, however, were still too faint to be detectable in all but the largest tumors. To improve the sensitivity enough to map the presence of deuterized lactate, the scientists developed combined experimental and image-processing approaches that enhanced sensitivity by more than an order of magnitude, enabling "deuterium MRI" to detect even very tiny amounts of these "doctored" lactate molecules.
The results of Frydman's deuterium MRI technique were crystal clear: Even low concentrations of deuterated lactate produced scans in which brightly lit-up regions showed millimeter-sized tumors, while the scan remained "dark" everywhere else. Frydman and his team also found that their approach was much more sensitive than a competing MRI technique that seeks to identify cancer by monitoring only the final step of the glucose digestion process in cancer cells.
Emphasizing that this work was performed on animal models and that his technological findings need to be confirmed in human patients, Frydman believes that deuterium MRI offers a new horizon for improved, early detection of pancreatic cancer. It does not, however, offer a cure.
"Future clinical studies, which we plan to start as soon as possible, could show that deuterium MRI is a lifesaving early-diagnosis modality for individuals possessing a genetic predisposition to this hideous disease," he states. "Even if the cancer is not caught in time, deuterium MRI will help measure rates at which the glucose-to-lactate conversion happens. This could provide a crucial metric for predicting the usefulness of certain treatments, or even determining whether a treatment is working. This could establish deuterium MRI as a preferred method for diagnosing hard-to-identify pancreatic tumors and choosing the treatment that will generate the best prognosis."
Science Numbers
Pancreatic cancer is the 12th most common cancer worldwide, but was the 6th deadliest in 2020; unless early detection methods are found, within a decade it is expected to become the deadliest cancer.
The study was led by Frydman and Dr. Elton T. Montrazi of the Weizmann Institute's Chemical and Biological Physics Department. Also participating in the research were Dr. Keren Sasson and Dr. Lilach Agemy, members of Scherz's lab.
Prof. Lucio Frydman is head of the Clore Institute for High-Field Magnetic Resonance Imaging and Spectroscopy and head of the Fritz Haber Center for Physical Chemistry. His research is also supported by the Rising Tide Foundation and the Harold Perlman Family.






