In a study published in Nature Aging on Nov. 26, a research team from the Shanghai Institute of Nutrition and Health (SINH) of the Chinese Academy of Sciences revealed that aging specifically impairs the generation of CD8+ tissue resident memory T cells (TRM) and thus compromises the antitumor defensive activity of aged CD8+ T cells.
With the aging process, the risk of developing cancer significantly increases. In recent years, it has been reported that immune aging has an important impact on tumor development. Immune aging is a degenerative change in the immune system that occurs with aging, leading to a decline in immune response and ultimately triggering diseases including tumors.
Within the immune system, CD8+ T cells are the main defensive adaptive immune cells protecting against tumor cells. However, the mechanism by which aging impairs the antitumor response of CD8+ T cells was not previously understood.
To investigate the intrinsic role of aging on CD8+ T cell function, Prof. XIAO Yichuan's team from SINH and collaborators isolated CD8+ T cells from young and aged mice and then adoptively transferred these cells into Rag1-/- mice. Single-cell RNA sequencing (scRNA-seq) of tumor infiltrating CD8+ T cells showed that the percentage of the TRM cell subpopulation decreased in the aged group.
To figure out how aging causes a decrease of CD8+ TRM cells, the researchers used multifactorial gene screening and found that high expression of the E3 ubiquitin ligase BFAR inhibited the production of TRM cells among CD8+ T cells derived from elderly individuals. Mechanistically, the E3 ligase BFAR suppressed cytokine-induced JAK2 signaling by activating JAK2 deubiquitination through the deubiquitinase USP39, thereby limiting downstream STAT1-mediated TRM reprogramming.
Based on the role of the key E3 ligase BFAR in CD8+ T cell function, the researchers applied deep learning combined with molecular simulation to screen a library of approximately 1,474,607 compounds. Through this process, they identified iBFAR2, a small molecule compound that inhibited USP39 ubiquitination and JAK2 deubiquitination, thus promoting STAT1 phosphorylation and TRM differentiation, which subsequently boosted antitumor immunity in aged mice.
In addition, researchers found that targeting BFAR could not only revive the antitumor activity of aged CD8+ T cells, but also enhance the sensibility of PD-1 blockade-based immunotherapy. PD-1/PD-L1 immune checkpoint blockade therapy has become a major weapon in fighting advanced tumors; however, most patients are refractory to the therapy or acquire resistance, especially those who are elderly.
In summary, this study revealed the role of aging in compromising the anti-tumor immune response of CD8+ T cells and the molecular mechanism involved. Further investigation of inhibitors targeting BFAR will promote the development of cancer immunotherapy in elderly patients and patients resistant to anti-PD-1 therapy.
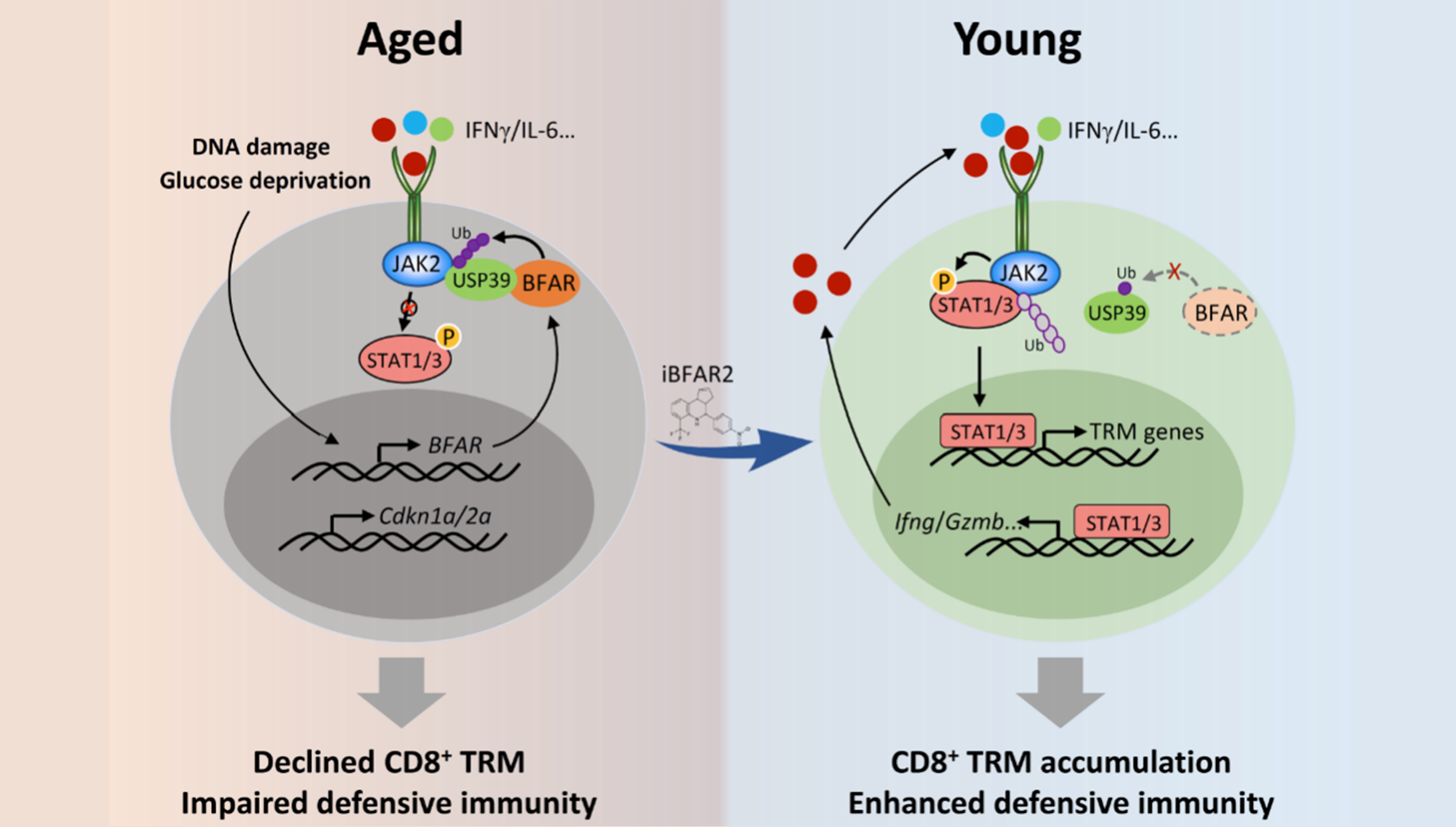
Working model of aging compromise CD8+ T cell-mediated antitumor immunity. (Image by Prof. XIAO's group)






