After an injury to the elbow, such as a fracture or dislocation, the joint may stiffen and be difficult to move, making everyday tasks such as eating, bathing or getting dressed challenging and painful. Treatment usually includes physical therapy and pain medication, but specific approaches that can best reduce the impact of the injury haven't been improved because little is understood about the mechanisms driving the motion loss.

Now, Spencer Lake, an associate professor of mechanical engineering and materials science in the McKelvey School of Engineering at Washington University in St. Louis, plans to study the effects of physical therapy and anti-inflammatory treatments in post-traumatic joint contracture in the elbow with a five-year $2.4 million grant from the National Institutes of Health (NIH). With collaboration from Ulugbek Kamilov, an associate professor of electrical and systems engineering and of computer science and engineering, he will incorporate machine learning tools to help shed light on how tissues within the joint respond to different types of treatments.
Lake, whose Musculoskeletal Soft Tissue lab studies injuries to joints, tendons and ligaments, will build on previous work that showed that movement of the joint soon after injury was found to reduce scar tissue formation and helped to preserve range of motion. Their recent preliminary data also showed that modulating the inflammatory response to injury may help improve healing.
In the new study, the research team plans to look at the timing, duration and intensity of the two treatment strategies that best prevent post-traumatic joint contracture. In addition, because joint contracture is known to involve both physical and biological mechanisms, the team will investigate whether combining treatments that target both mechanisms will be more effective than either treatment alone. To do this, they will integrate machine learning analyses to study the effects of various treatments in a rat model of elbow injury.
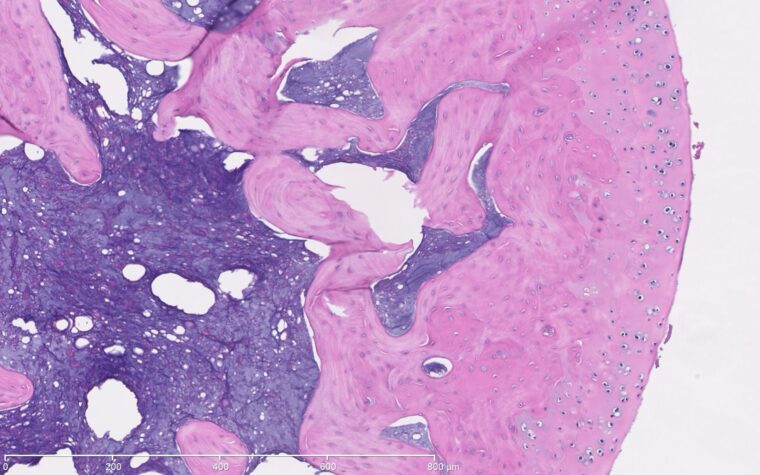
"We get high-resolution images of tissue histology and also acquire magnetic resonance images," Lake said. "These are rich sources of data that provide a lot of insight, but there are also likely additional patterns that we're not seeing currently. We're developing machine-learning algorithms to try to detect these patterns and extract more information from these images than we can visually, then link the data modalities together."
Lake's ultimate goal is to detect how different tissues are changing following injuries and treatments so that they can determine which therapeutic parameters are most critical to optimize tissue healing and functional recovery.
"We want to determine whether the treatments are effective and improving tissue-level properties or whether the tissues are still injured," Lake said. "Ideally, if we could correlate the data from the MRI, which is noninvasive, with the data we're getting from more invasive tissue-analysis techniques, that would lend better toward translation to humans."
Lake's lab will be working with Benjamin Zmistowski, MD, an assistant professor of orthopedic surgery at WashU School of Medicine, who will perform surgical aspects of the study and provide perspective from experiences treating human patients with elbow injuries. Renee Ivens, an associate professor of physical therapy, will share clinical insights related to physical therapy approaches following joint injuries in humans.
"Physical therapy in rats is obviously different than in humans, but if we can identify key parameters like intensity, time of initiation, frequency and duration, which are most critical for preserving joint motion, we think those concepts will translate to improved treatments for humans," Lake said. "In addition, if we can provide the appropriate level of physical therapy and also modulate the level of inflammation to get synergistic therapeutic effects, we're optimistic that we could translate key parameters that guide such a combined approach to humans."
Previously, Lake's team had the rats run on a treadmill to provide physical therapy to the elbow joint, but beneficial effects were limited. Now, they have transitioned to running wheels that allow more frequent activity across a wider range of joint motion; early results have shown promising improvements.
"We built instrumented wheels that require the animals to exert more joint extension in order to run," Lake said. "The instrumented wheels allow us to track every rotation so we can get precise measurements of how much physical activity they're getting, which we can then correlate to metrics indicative of their healing and recovery."
However, post-traumatic joint contracture is known to have both physical and biological causes, so physical therapy alone may not be enough to prevent pain and stiffness from setting in after injury. To address this, Lake's team partnered with Matt Bersi, an assistant professor of mechanical engineering and materials science at McKelvey Engineering, to conduct experiments revealing that one of the key players in contracture development is T cells, a type of immune cell that sends signals to help control the immune system's response after an injury has occurred.
"In addition to trying a broad anti-inflammatory drug, we're also going to be targeting a cytokine (a signaling molecule for cell communication) called IL-17A that T cells commonly produce to see if just that specific treatment is sufficient," Lake said.
If successful, this cytokine-based approach will elucidate a key mechanism of joint contracture and could motivate treatments that avoid unintended side effects that are possible with broader drug approaches.
"If we could block the main contributor to excess tissue deposition with biological treatments and loosen stiff connective tissue with physical therapy, then the regular healing response could proceed normally without long-term loss of motion and disability," he said.
Originally published on the McKelvey School of Engineering website






