Environmentally friendly and efficient: recycling lithium-ion batteries in neutral solution
A new strategy for recycling spent lithium-ion batteries is based on a hydrometallurgical process in neutral solution. This allows for the extraction of lithium and other valuable metals in an environmentally friendly, highly efficient, and inexpensive way, as a Chinese research team reports in the journal Angewandte Chemie. The leaching efficiency is improved by a solid-solid reduction mechanism, known as the battery effect, as well as the addition of the amino acid glycine.
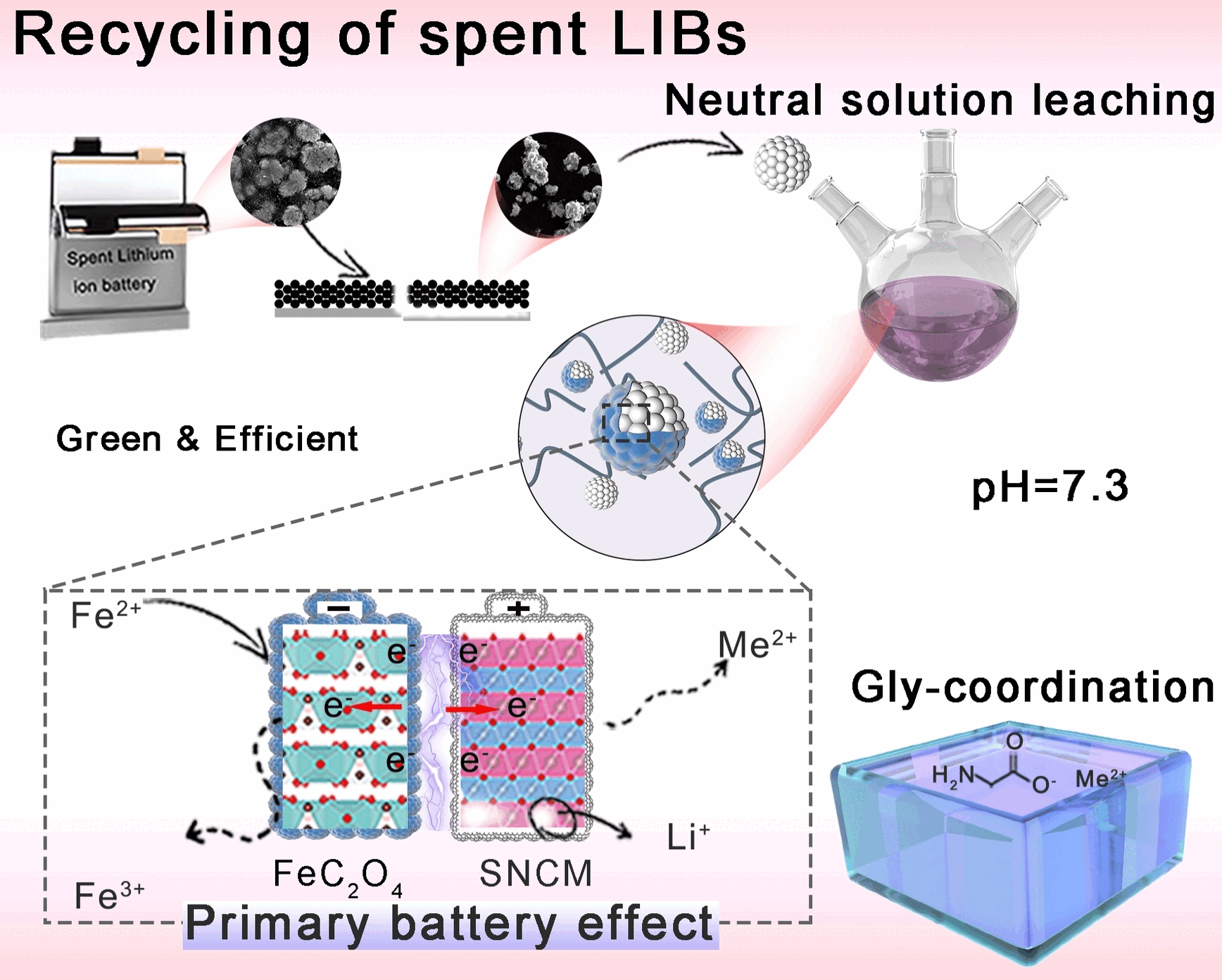
© Wiley-VCH, re-use with credit to 'Angewandte Chemie' and a link to the original article.
Lithium-ion batteries not only power our mobile phones, tablets, and electric vehicles, they are also increasingly important as storage for volatile renewable energy. As they become more widely used, the number of spent batteries keeps increasing. Their recycling is promising, having the potential to reduce environmental impact while extracting raw materials such as lithium, cobalt, nickel, and manganese for the production of new rechargeable batteries. Current hydrometallurgical methods for the reprocessing of spent lithium-ion batteries are based on acid or ammonia leaching processes. However, excessive and repeated use of acids and bases increases the environmental impact and safety hazards. A pH neutral process would be safer and more environmentally friendly.
To come up with a neutral approach, the team led by Lei Ming and Xing Ou at Central South University in Changsha, Zhen Yao at Guizhou Normal University, and Jiexi Wong at the National Engineering Research Central of Advanced Energy Storage Materials had to reach deep into their bag of tricks because the aggressive reagents required for classical leaching processes are not easy to replace.
The first trick: They constructed "micro batteries" in situ. These help to break up the spent cathode material from the batteries-lithium-coated nickel cobalt manganese oxide (NCM). The NCM particles are mixed with an iron(II) salt, sodium oxalate, and the amino acid glycine in a neutral liquid. This results in the deposition of a thin, solid layer of iron(II) oxalate on the particles. This "shell" acts as an anode while the NCM cores act as the cathode (battery effect). This direct contact allows for easy electron transfer. The coating also hinders deposition of undesired byproducts on the particles. The battery effect drives an electrochemical reaction in which the iron(II) ions are oxidized to iron(III) ions and oxygen ions from the oxidic NCM particles are reduced to OH- ions with water. This breaks up the NCM layers, releasing the lithium, nickel, cobalt, and manganese ions they contain into the solution. In the second trick, these ions are "trapped" in complexes by the glycine. Glycine also has an additional task: it buffers the pH value of the solution, maintaining a neutral range. Within 15 minutes, it was possible to leach 99.99 % of the lithium, 96.8 % of the nickel, 92.35 % of the cobalt, and 90.59 % of the manganese out of spent cathodes.
This efficient leaching in neutral solution could open new pathways to the realization of large-scale, environmentally friendly recycling of spent batteries. Barely any harmful gases are produced, and the glycine effluent is suitable for use as a fertilizer. This process uses significantly less energy and costs less than conventional methods.
(3404 characters)
About the Author
Dr. Xing Ou is a Professor at the School of Metallurgy and Environment, Central South University. His primary research focuses on advanced materials for energy storage and environmental applications, with a particular emphasis on the development of sustainable technologies for resource recovery and pollution control. He has been actively involved in numerous national and international research projects, contributing significantly to the field of environmental metallurgy.






