At the beginning of the 20th century, the invention of X-ray imaging provided a leap of knowledge in medical science. Since then, we can see how our body's bones work, bringing numerous new treatments to light. Now, a similar approach using neutron imaging makes it possible to visualize the internal functioning of redox flow batteries - a type of battery mainly used for large-scale storage in solar and wind energy systems. Being able to see inside these batteries offers new possibilities for improving them.
An international collaboration - between TU/e, the Massachusetts Institute of Technology (MIT), and the Paul Scherrer Institute in Switzerland (PSI) - led by TU/e researcher Antoni Forner-Cuenca, developed this new method using neutron imaging. The breakthrough provides extraordinary moving images (see video further down) that help understand redox flow batteries' inner workings.
Curiosity-driven research across disciplines
More importantly, the images provide inspiration and guidelines for new ideas and solutions. More directly, the method can aid the development of redox flow batteries, although the new imaging technique devised by Forner Cuenca's team may also help other scientific disciplines move forward. "Our method is the result of experimenting on and borrowing from different fields. It is an exciting example of the importance of curiosity-driven research across disciplines."
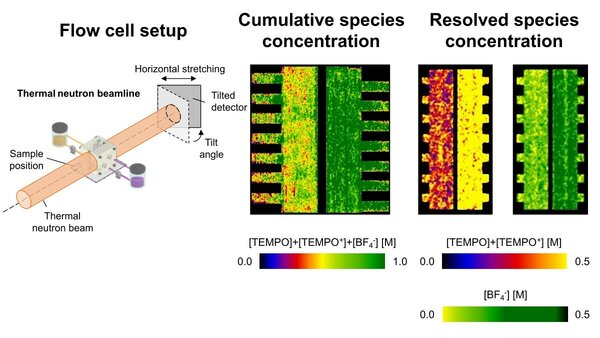
Neutron radiography plays a crucial role in the research entitled 'Quantifying concentration distributions in redox flow batteries with neutron radiography'. Forner Cuenca learned a lot about this imaging technique during his PhD training, which started in 2013 at the PSI. Then, in 2017, he performed postdoctoral research at MIT, where he learned about redox flow batteries. That's when the light bulb went on in his head.
System remained a black box
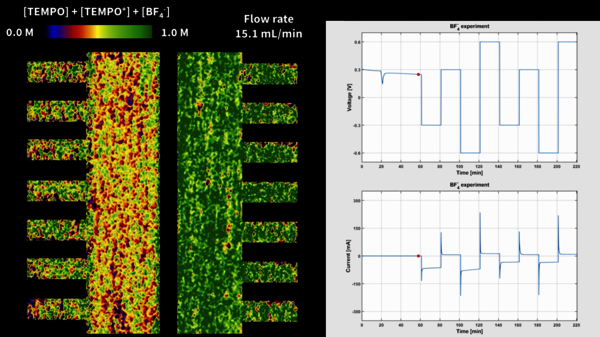
"Inside the flow battery, there are moving fluids - the so-called electrolytes. An electrical current flows through the cell when the battery runs in charge or discharge. Consequently, ions and redox molecules in the electrolyte start to move in different directions, resulting in changes in the concentration of molecules. That movement determines the battery's performance and durability, but to date, the system has remained a black box. The ability to look inside a working battery and visualize concentration distributions would enormously improve our understanding of the system."
So, a key factor in how that battery works remained uncharted territory, which got Forner Cuenca thinking. "Our bodies are also mostly composed of fluids, namely water. X-rays pass through that and interact with heavier elements in your bones, allowing you to see them without cutting open a body. Neutrons work the opposite way: they pass through the battery casing materials easily but interact strongly with the molecules in the liquid electrolytes."
A new application of existing science
"Using this fundamental property of neutrons interacting with certain molecules, we are using neutron radiography for the first time to look at concentrations of molecules in flow batteries." A new application of existing science, in other words. "That technique itself is not new; it is already used by museums, for example, to see what historical objects are made of without damaging them. But now we can also use it to visualize moving fluids, as in redox flow batteries."
The method used by Forner-Cuenca and his team is still much more laborious than X-ray photography, though, and similar to stop-motion animation. "To track in real time how the concentration of liquids changes in the battery, we continuously take pictures every 30 seconds of the collection of neutrons that travels through the battery. We piece those pictures together, so to speak, providing us with a video that shows how the concentration changes during battery operation."
Measuring for 24 hours in 10 day shifts
These experiments were conducted at the neutron source of the PSI. A collaborative team of three PhD students was in charge of the experiments with Forner-Cuenca - Remy Jacquemond, Maxime van der Heijden, and Emre Boz, who are now successfully graduated doctors. Since the experiments were intense, the team measured for 24 hours in various shifts for around 10 days to maximize productivity.
"Having the opportunity to use neutrons is an extraordinary experience; we only get to use equipment like that once every two years, on average. The PSI (the Paul Scherrer Institute in Switzerland, where the experiments took place, ed.) has an annual international experiment competition ranked by importance. We have been privileged to perform four successful experiments."
"In terms of effort and expertise, this project was challenging, and having three PhD students collaborating was essential for its success. I am very proud of these three colleagues, who worked hard and collaborated as a true team. It shows the strong value of working in teams, both in our research team and with international collaborators at PSI and MIT."
Plenty of areas for improvement
According to Forner Cuenca, visualizing fluid action in Redox flow batteries is important for several reasons. "Of course, understanding processes occurring inside the battery means that we can develop better-performing systems that work more efficiently and have longer lifetimes. Therefore, since they are mainly used to store renewable energy from solar and wind, we hope to contribute to the energy transition." There are still plenty of areas for improvement, as Forner Cuenca explained in this earlier article on our website.
However, as with any new technology, it also offers other possibilities in the future. "For example, chemical reactors are used to make all kinds of products such as plastics, cosmetics, and medicines. Since our method enables visualization of organic molecules in a solution, we anticipate that other industrial applications can benefit from our imaging technique."
These new insights may, in turn, lead to completely different methods or ideas. "That's what excites me the most: fueling curiosity. After all, this is how we developed this new methodology. Collaborative research and curiosity-driven ideas are two critical elements of scientific discoveries. Supported by an ERC grant that embraces blue-sky projects, we were able to develop this method and we have many new ideas to pursue in the future."
Reference:
Jacquemond, R.R., van der Heijden, M., Boz, E.B. et al. Quantifying concentration distributions in redox flow batteries with neutron radiography. Nat Commun 15, 7434 (2024). https://doi.org/10.1038/s41467-024-50120-7