The function of a key sleep-promoting protein in the mouse brain depends on how it is chemically tagged, RIKEN researchers have found1. This discovery could lead to new medications for inducing and maintaining sleep.
The sleep-wake cycle is a physiological function that determines the rhythms of when we fall asleep and wake up. While the cycle has been much studied, there is still a lot that we don't know about its molecular mechanisms.
Previously, Hiroki Ueda from the RIKEN Center for Biosystems Dynamics Research and his colleagues had identified sleep-promoting proteins whose activity could be modulated by phosphate tags. Based on this observation, they proposed that this tagging of phosphate groups-or phosphorylation-regulates the sleep-wake cycle.
Now, by focusing on one sleep-promoting protein in particular-a neuronal enzyme called CaMKIIβ-Ueda and his team have shown how different phosphorylation patterns on this critical protein in mice can control different sleep induction and maintenance processes in their brains (Fig. 1).
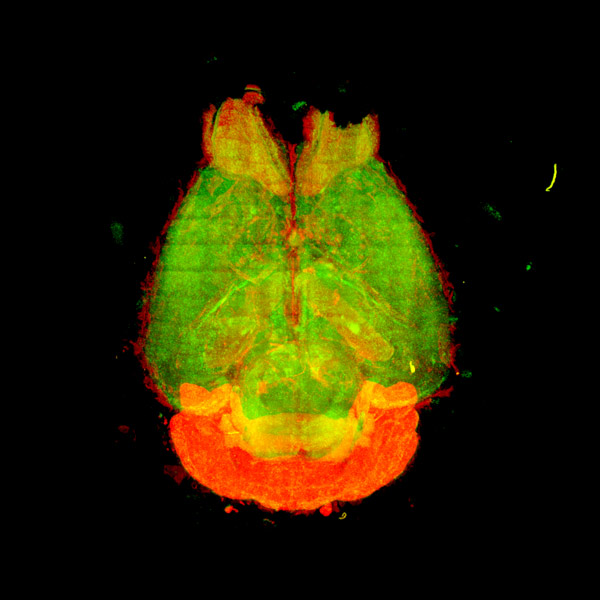
Figure 1: To investigate the effect of CaMKIIβ on sleep, RIKEN researchers measured the expression of the neuronal enzyme in different regions throughout the mouse brain. Reproduced from Ref. 1 and licensed under CC BY 4.0 © 2022 D. Tone et al.
For example, mice with a mutant form of CaMKIIβ that mimics an 'always on' phosphorylation state tended to transition more readily from wakefulness to slumber. In contrast, mice with mutations that resemble a different multisite phosphorylation pattern tended to stay asleep longer, and the transition to waking up was supressed.
"These findings are consistent with our proposal that sleep-promoting enzymes such as CaMKIIβ can store information about wakefulness in the form of phosphorylation events," explains Ueda. "In turn, control of sleep and wakefulness can be carried by the state of a specific enzyme in the brain."
The results could potentially have practical applications, since they highlight new potential avenues for developing sleep-enhancing medications.
Ueda and his team showed that the inhibition of CaMKIIβ could dramatically reduce sleep duration in the mice to less than 10 hours, while the induction of CaMKIIβ could greatly extend sleep duration to more than 15 hours (mice tend to sleep a lot more than humans do).
"Pharmacological agents that similarly augmented CaMKIIβ activity may have the same sleep-promoting effects," says Daisuke Tone, a member of Ueda's lab. "And by tweaking phosphorylation specifically, drug companies could develop different CaMKIIβ-targeted therapies that either put people to sleep or keep them asleep longer."
The latter approach has rarely been tried before. "But it may lead to the development of sleep-maintenance drugs, which could offer a new therapeutic strategy for sleep disorders," says Tone.
(From right) Hiroki Ueda, Daisuke Tone and Koji Ode © 2023 RIKEN






