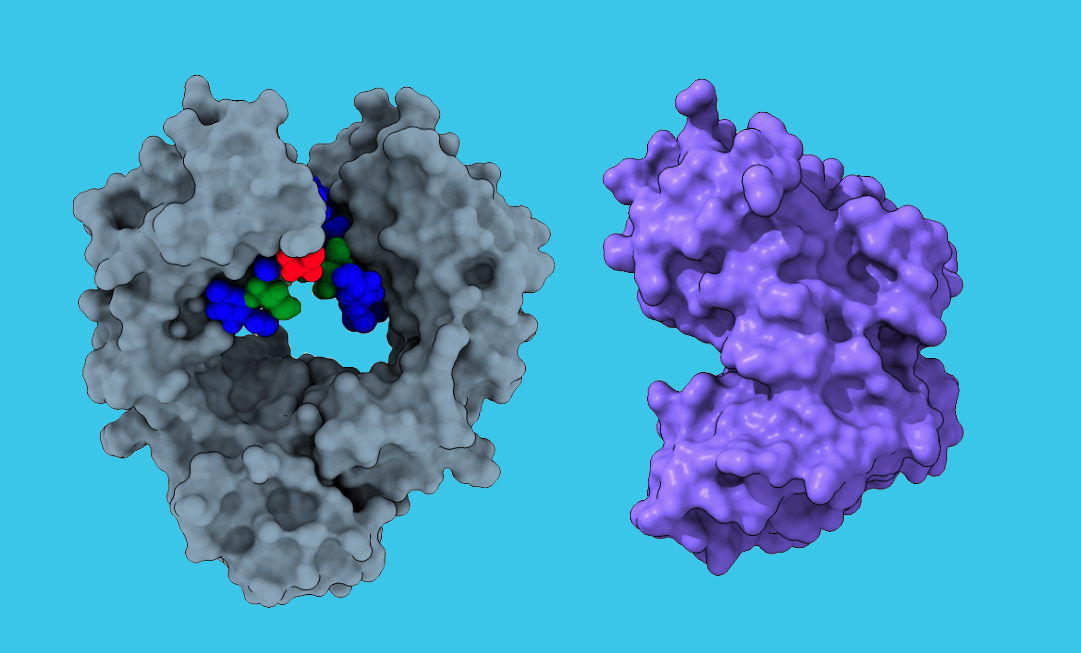
To interact with specialized receptors on leukocytes, an antibody (left) depends on a glycan (multicolor). The endoglycosidase CU43 (right) binds to the antibody and cleaves off the glycan, which kneecaps its ability to interact with a leukocyte and enhances its pathogenic activity. (Ravetch lab)
Researchers have discovered a powerful new enzyme that could one day transform the treatment of myasthenia gravis and other autoimmune diseases. Their findings, published in Cell, suggest that this enzyme outperforms existing drugs in mouse models, working more effectively at lower doses and setting the stage for future clinical trials in humans.
"The potency of this enzyme is quite remarkable when compared to current treatments for autoimmune diseases, and thus warrants consideration for further development for the treatment of this important class of diseases," says Jeffrey Ravetch, the Theresa and Eugene M. Lang Professor at Rockefeller, who contributed to the research led by Emory University.
A debilitating chronic immune disorder in which the body's own antibodies interfere with communication between muscles and nerves, myasthenia gravis results in muscle weakness and, in severe cases, difficulty breathing. It's one of a family of IgG-mediated pathologies, which arise from the body's inability to keep certain antibodies under control.
Since current treatments for this class of diseases, such as IVIG, anti-C5 antibodies, and FcRn inhibitors, focus on reducing or neutralizing IgG antibodies, the Emory team explored whether bacterial enzymes could offer a new approach. After screening a database of similar enzymes, they flagged a particular subset, produced by Corynebacteria, which disables IgG antibodies by targeting a specific sugar molecule, or glycan, within the antibodies' Fc regions. They then tested the most promising enzyme, CU43, in mouse models that had been genetically modified to mimic the function of human immune cells and human IgG pathologies.
The collaboration between Emory and Rockefeller drew heavily on Ravetch's expertise in studying the Fc receptors of antibodies and engineering so-called "humanized" mouse models to better understand and treat autoimmune diseases. "Removing the glycan from the Fc region of autoantibodies with the enzyme discovered by the Emory group appears to be an effective strategy for reducing their pathogenicity," Ravetch says.
Indeed, CU43 ultimately outperformed current myasthenia gravis treatments in mice, reducing symptoms more effectively at much lower doses-it took 4,000 times less enzyme to achieve the same effect. "The enzymes we discovered can modify antibodies in such a way that they no longer cause disease," says lead author Eric Sundberg of Emory University.
The results highlight CU43's potential to treat not only myasthenia gravis but also other IgG-mediated diseases. For instance, CU43 was also shown to be effective in mitigating autoimmune hemolytic anemia and antibody-dependent enhancement of viral diseases such as dengue. And if the mouse data is any indication, CU43 could be effective at lower doses, resulting in fewer side effects and expanded treatment options for patients. "Importantly, unlike current treatments for autoimmune diseases that often have immunosuppressive effects, CU43 represents a more targeted approach that specifically renders pathogenic antibodies inactive, while preserving important functions of protective antibodies necessary for host defense against pathogens," says Stylianos Gournazos, a research associate professor in Ravetch's lab and co-author of the paper.
"We hope to leverage these promising results in mice to move this enzyme rapidly into clinical trials in humans," Sundberg says. "It could potentially be used to treat a wide range of autoimmune diseases and other IgG-mediated pathologies, offering a powerful new approach to treating conditions that currently have limited therapeutic options."






