By Alan Dove | Portraits by Jörg Meyer
In the 16th century, influential Swiss physician and alchemist Paracelsus published a recipe for artificially creating a homunculus-a miniature human-that would bypass the normal reproductive process. Though the recipe failed, the metaphor has thrived in speculative fiction and philosophy ever since.
Indeed, biomedical researchers have relied on their own homunculi for decades, either culturing human cells in petri dishes or manipulating animals to mimic specific aspects of clinical conditions. Those two strategies have undergirded most of the progress in medical science since the mid-20th century, but they suffer from major limitations. As a result, treatments that work beautifully in cell culture and animal models often fail in the clinic.
A better approach may be just around the corner, thanks to a series of scientific breakthroughs over the past several years.
First, researchers discovered that by activating a few specific genes, they could induce fully differentiated cells to reverse their developmental clocks, becoming pluripotent stem cells capable of re-differentiating into another tissue type entirely. An easily obtained sample of human skin cells, for example, can be turned into an endless supply of liver, heart, or brain cells genetically identical to the donor.
More recently, investigators have learned how to grow these induced pluripotent stem cells into complex three-dimensional structures, such as organoids, engineered tissues, and organs on a chip. These miniature models of patients' organs are fast becoming standard research tools in laboratories at Columbia and worldwide.
Skin Deep
Many researchers use patient skin cells as a starting material for creating organoids, but for Yvon Woappi, PhD, assistant professor of physiology & cellular biophysics, skin is also the endpoint. "A lot of the diseases of the skin are thought of as cosmetic, but we think of the skin as a microcosm of the entire body, because there you virtually have all of the cell types that are found in the body," says Dr. Woappi.

Yvon Woappi
Skin is one of a few parts of the body in which stem cells persist throughout life. With millions of skin cells dying and falling off each day, the specialized, self-renewing skin stem cells give rise to a continuous supply of fresh replacements. Dr. Woappi became interested in that process while studying human papilloma virus-HPV-which can persist in the skin for decades and cause either benign warts or deadly cancers.
To determine where HPV replicates, Dr. Woappi and his colleagues developed organoid culture systems in which to grow it. These "skinoid" cultures revealed that the virus can infect the skin stem cells, helping it maintain a persistent infection even as the descendants of those cells die off.
Skinoids have another useful feature: When transferred from growing in three-dimensional space in suspension to growing in two-dimensional layers in petri dishes "that mechanical switch cues skin cells to proliferate and behave as if a wound has been generated," says Dr. Woappi. "Now we have a high-throughput system to study wound healing completely in a dish." His lab plans to use that system to investigate how wound healing differs between individuals by comparing skinoid cultures derived from thousands of different donors in the hope of identifying ways to make the healing process more reliable.
Though the initial results from skinoids have already shown promise, Dr. Woappi's team is trying to improve the system. The lab derived original skinoids directly from the naturally occurring stem cells in skin samples, but those partially differentiated cells bring their own baggage in the form of epigenetic changes unique to an individual's life history. "Each sample comes with its own sort of reaction to the tissue culture environment," says Dr. Woappi. As a result, fewer than half of the patient samples the lab gets yield usable skinoids.
To address that, the researchers are trying to generate induced pluripotent stem cells from the skin samples, winding back their development to a much earlier state before re-deriving fresh skin stem cells. That should eliminate the epigenetic variability that currently limits the system and yield more reproducible skinoids for direct comparisons between patients.
Dr. Woappi is also interested in pursuing clinical applications for skinoids. "We're able to generate about 200-fold greater amounts of keratinocytes than were in the original sample and so we are thinking about actually using this as a strategy to expand epidermal stem cells for transplantation to burn victims in the clinic," he says. Though he cautions that such applications are at least several years away, Dr. Woappi is optimistic about skinoids' potential. "This is a very exciting interest of ours, to be able to use this therapeutically."
Down the Hatch
Organoids are helping researchers get around the limitations of traditional animal and cell culture models for studying other tissues, too. Hiroshi Nakagawa, MD, PhD, has been using mouse models for the past three decades to study diseases of the esophagus. "But in the last five or six years, the newly emerging organoid technologies appear to be very robust," says Dr. Nakagawa, associate professor of medicine and a member of the Herbert Irving Comprehensive Cancer Center.

Hiroshi Nakagawa and Cecilia Martin
One major advantage of organoids is their rapid growth. Dr. Nakagawa explains that his team can turn a small clinical specimen into an esophageal organoid in a couple of weeks. Because the resulting organoid is derived directly from a specific patient, it provides an excellent model of that patient's biology. "One of the greatest advantages is that we can recapitulate pathological features and drug response of a patient's tumor in a test tube in a very quick way, and then we can test a variety of drugs for customized medicine," says Dr. Nakagawa.
While organoid-based chemotherapy customization is not yet ready for routine clinical use, organoids are already yielding a trove of new findings about the biology of esophageal cancers, which are among the deadliest of human cancers. "We want to know how the environmental risk factors, such as alcohol and smoking, could influence these cell populations to undergo malignant transformation," says Dr. Nakagawa. He and his colleagues have seen major changes in gene regulation in different types of esophageal cells in response to such environmental insults and have also identified some of the molecular pathways responsible for both tumor growth and drug responses in the esophagus.
In collaboration with Columbia surgeons and oncologists, Dr. Nakagawa's lab is creating organoid systems to mimic other types of tumors, including some head and neck (oral and throat) cancers, skin cancer, and cervical cancer of the uterus. That has allowed the scientists to study many aspects of cancer development that weren't accessible with traditional cell or animal models. "Organoids permit us to grow not only normal and cancer cells, but also the precancerous cells. In the past it was very difficult to grow those," says Dr. Nakagawa. Following the development of tumors through the precancerous stage is revealing previously unknown changes, which should help identify new markers for early diagnosis and potential treatment targets.
Organoids with tumor cells in different stages of oncogenesis feature in Dr. Nakagawa's collaboration with pathologists and bioinformatics experts at Columbia. That team hopes to develop artificial intelligence algorithms that will help pathologists score clinical samples more reliably and predict therapy outcomes.
"Organoids permit us to grow not only normal and cancer cells, but also the precancerous cells."
Dr. Nakagawa's team runs the organoid and cell culture core, which gives other researchers across the university access to these powerful experimental systems. Part of that core's mission is to train investigators to derive and maintain organoid cultures. "It's really just like learning any other new cell line when you start to culture it," says Cecilia Martin, senior staff associate and manager of the core. Techniques for maintaining most organoids are fairly straightforward, so "anyone with cell culture experience shouldn't be too intimidated to try to learn organoids."
If We Only Had a Brain
Other researchers are using the technology to simplify organs that are too hard to study intact. "The brain is this massively complex system so we focus on a region of the brain, or even simpler than that, a circuit," says Chris Makinson, PhD, assistant professor of neurological sciences (in neurology and the Center for Translational Research in Neurodevelopmental Disease). Dr. Makinson's team uses organoids representing specific brain circuits involved in epilepsy and similar neurological conditions, allowing researchers to probe the pathology of these diseases much more deeply than they could by looking at human brain scans.

Chris Makinson
Though they're much less complex than whole brains, the brain organoids Dr. Makinson grows can often replicate many of the processes that drive neurological diseases. "Probably all brain disorders are circuit disorders at some level, so having the tools to read out the activity of the circuit is really key if you want to understand what's going on," says Dr. Makinson.
Just a few years ago, researchers were thrilled to be able to generate any kind of neurons from induced pluripotent stem cells, but as the field has matured, the techniques have become more sophisticated. "By adding additional factors to the stem cells we can cause them to not just become brain, but to become cortex or thalamus or hippocampus or cerebellum or spinal cord. Then you've constrained how many different cell types you're going to get in the brain organoid," says Dr. Makinson. That yields more consistent organoid structures, which allows him to compare pathological and healthy brain circuits more reliably.
Gene editing technology has made organoid experiments even more useful. One major strategy Dr. Makinson and his colleagues have pursued starts with donor cells from patients with epilepsy caused by specific gene mutations. They then derive brain organoids from the cells as well as identical cells that have been genetically edited to fix the relevant mutation. "So you have your patient line, and then you have your patient corrected line, and you can look for differences between those two different lines," says Dr. Makinson. The gene-edited "twin" provides a powerful control for distinguishing normal from pathological nerve interactions.
By delaying the gene editing step, Dr. Makinson's team is trying to identify strategies to treat or prevent epilepsy. In those experiments, the investigators allow their brain organoids to mature for several months, then apply gene therapy to the differentiated cells to see if they can correct the organoid's pathology.
The rapidly increasing power of organoid studies has allowed labs like Dr. Makinson's to cut back significantly on their reliance on animal models. "Mice are still extremely powerful models by comparison right now, but I think that in five to 10 years, we might be at a place where we can go from understanding a mechanism to a clinical setting without the use of mice, or maybe very limited use of animals," says Dr. Makinson. Indeed, the FDA recently changed its longstanding policy mandating animal studies for new drug applications, paving the way for even greater reliance on organoids.
The Sum of Our Parts
The FDA's policy change followed years of other government initiatives to improve preclinical model systems, an effort some Columbia faculty have helped drive. "About 10 years ago, the National Institutes of Health invited the community to start thinking if what we have learned in the area of regenerative medicine could be helpful to build in vitro laboratory models of development and disease," says Gordana Vunjak-Novakovic, PhD, University Professor and the Mikati Foundation Professor of Biomedical Engineering.

Gordana Vunjak-Novakovic
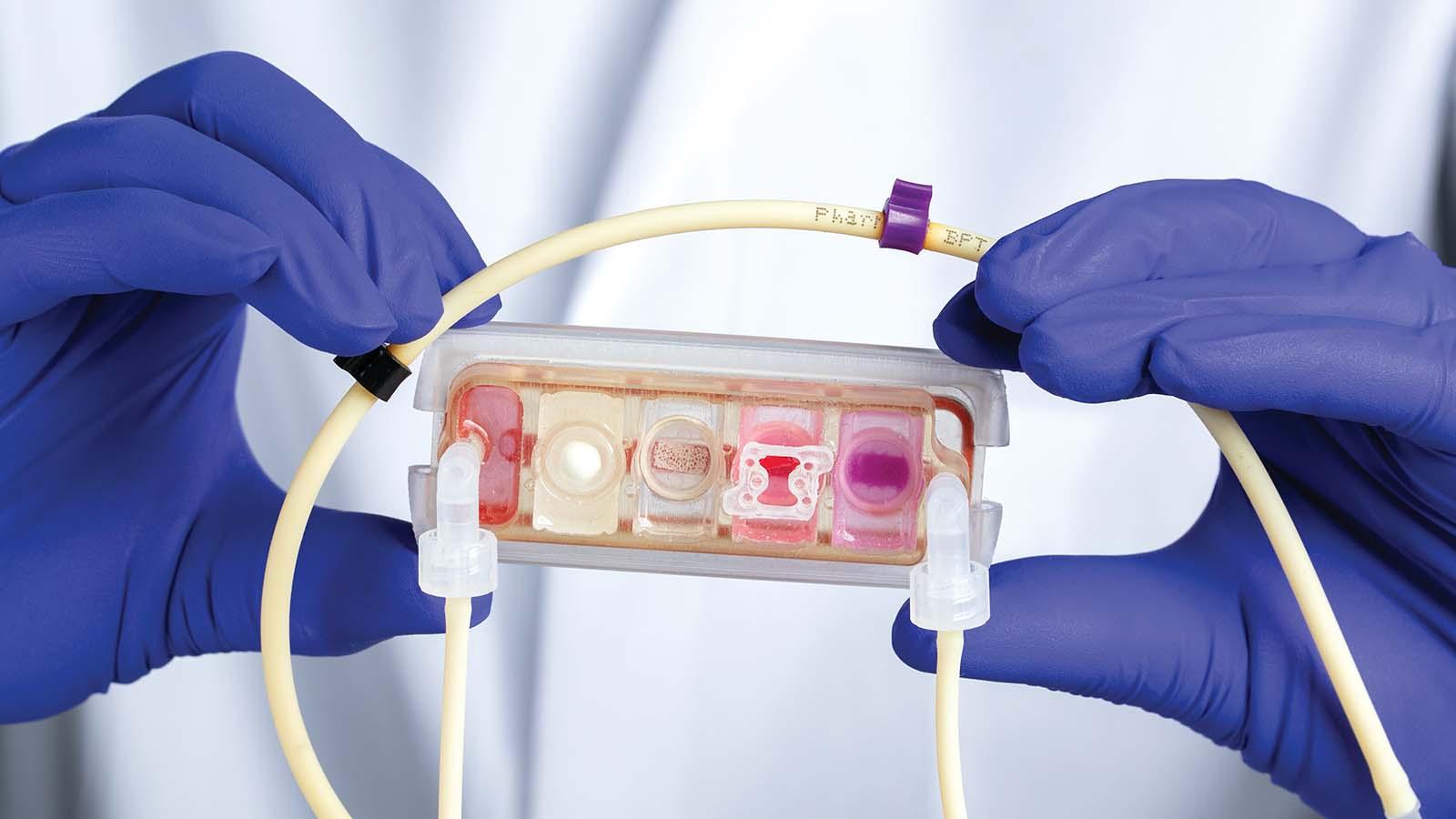
Dr. Vunjak-Novakovic and her lab embraced the challenge. With a background in chemical engineering, she has pioneered the development of the "organ on a chip" model, which combines tissue engineering with fabrication technologies borrowed from the semiconductor industry. That approach allows researchers to create complex three-dimensional structures such as miniature hearts, livers, and other organs.
"We are also interested in combinations. For example, we would study the heart muscle, but then this heart muscle is in the body communicating with the immune cells, so we plug in a bone marrow module to serve as a source of these immune cells," says Dr. Vunjak-Novakovic. Drug metabolism studies might include a liver module as well. To connect the miniature organs, she fabricates channels in the chip to carry blood between them, just like a circulatory system.
Though the components could be combined into a nearly complete homunculus, the investigators instead select the parts most relevant to each project. "You'll always try to make the simplest possible model that is complex enough and accurate enough to study your question," says Dr. Vunjak-Novakovic.
While the ability to perform patient-specific clinical studies on well-defined organoid models promises to make medical research both more reliable and faster, Dr. Vunjak-Novakovic is clear-eyed about the field's looming challenges. "It's incredibly important that we are mindful about what we can do and cannot do and that we very rigorously and systematically address the challenges and limitations of these systems," she says. In particular, her lab focuses on validating results from their multi-organ chips by comparing them directly to clinical findings.
Another challenge is in processing the immense quantities of data the new systems can produce. Because it's possible to monitor everything from individual cells' growth to changes in expression levels of every gene in the genome throughout an organoid, scientists working with these systems often generate far more data than they can analyze.
"It's incredibly important that we are mindful about what we can do and cannot do and that we very rigorously and systematically address the challenges and limitations of these systems."
More complex multi-organoid chips can be hard for some researchers to use. "Clinicians and biologists would normally not operate hardware, they just are used to working in cell culture dishes, so something we are working on now very, very actively is to redesign these artisanal devices into something that is as easy to use as a cell culture dish while maintaining this tremendous functionality," says Dr. Vunjak-Novakovic.
The crucial tool for overcoming those challenges may be as simple as increased collaboration among scientists. "I'm very optimistic, because this is the era of collaboration. We are collaborating more effectively than ever before in the history of sciences, and I think that this is the key," says Dr. Vunjak-Novakovic.






