AXL and EGFR inhibitors combined hold promise in fighting certain head, neck, and lung cancers
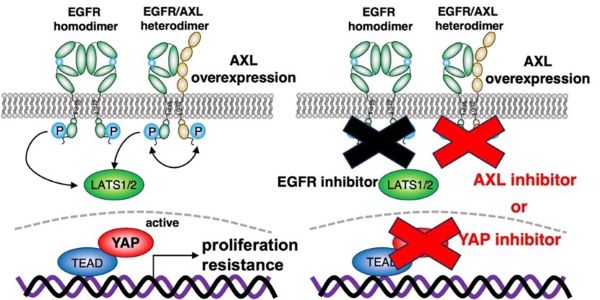
Schematic of mechanistic mechanism by which AXL activates YAP
Scientists from from Hiroshima University Hospital clarified the mechanism by which AXL heterodimerizes with EGFR to activate the EGFR-LATS1/2-YAP axis, resulting in proliferation and resistance against EGFR-targeted drugs (left). These results suggest that the combination of the drug targeting AXL or YAP with EGFR targeted therapy may be beneficial for preventing cancer recurrence and progression acquired by YAP re-activation. (Toshinori Ando, Hiroshima University Hospital)
An international team of researchers has demonstrated that a combination of inhibitors may suppress tumor growth and prevent relapse in patients with certain cancers, including head and neck squamous cell carcinoma and lung adenocarcinoma. Their findings support the future development of innovative therapeutic approaches targeting these cancers.
The team's work is published in the journal Oncogene on August 17, 2023.
Scientists know that in humans and other mammals, the Hippo signaling pathway plays a key role in the rapid increase of cells that occurs with cancers in the body. Yes-associated protein 1, or YAP, is a protein that is critical in regulating the progression of tumor growth, and it plays an important role in the beginning and spread of a variety of cancers. When the Hippo pathway is dysregulated, it triggers the activation of YAP and this contributes to head and neck squamous cell carcinoma. Both the Hippo pathway and YAP have attracted attention as signaling pathways that regulate cancer cell characteristics.
Epidermal growth factor receptor, or EGFR, is a protein on cells that contributes to their growth. When a mutation occurs in the gene for EGFR, it can grow too much, leading to cancer. EGFR is frequently amplified and highly overexpressed in head and neck squamous cell carcinoma, and mutated and activated in lung adenocarcinoma. So the EGFR inhibitor, a drug that blocks the cancer's growth, is used as a targeted therapy in fighting these cancers.
In earlier work, the research team clarified the mechanism by which EGFR activates YAP through the Hippo pathway. However, EGFR-targeted monotherapy has shown a low response rate. Based on this evidence, researchers believe that EGFR inhibitors may temporarily inactivate YAP, but when YAP is re-activated, it increases resistance to the EGFR inhibitors used to fight the cancer. Scientists do not yet fully understand how the YAP is re-activated.
The team focused their current study on AXL, a receptor-type tyrosine kinase. They set out to clarify the mechanism that causes the cancer cells' resistance to EGFR inhibitors, specifically focusing on the novel regulatory mechanism of YAP by AXL. Receptor-type tyrosine kinases like AXL play an important role in cell processes. When it is working properly, AXL is mainly expressed in immune cells, and does the work of removing dead cells and controlling the duration of immune responses. But when AXL becomes dysregulated, they can contribute to cancers, including lung adenocarcinoma, acute leukemia, and head and neck squamous cell carcinoma.
The team used comprehensive transcriptional analysis and in vitro experiments in their study. With this research, the team clarified that AXL stimulates YAP through a novel mechanism when AXL combines with EGFR. This combination activates YAP via the EGFR-LATS1/2 axis. LATS1/2, or large tumor suppressor kinases, are important members of the Hippo pathway. The team determined that the combination of AXL and EGFR inhibitors working together inactivates YAP and suppresses the viability of head and neck squamous cell carcinoma and lung adenocarcinoma cells.
"The combination therapy targeting both EGFR and AXL or YAP simultaneously may effectively suppress tumor growth and prevent resistance and relapse in patients with EGFR-altered cancers, including head and neck squamous cell carcinoma and lung adenocarcinoma," said Toshinori Ando, assistant professor at the Center of Clinical Oral Examination, Hiroshima University Hospital.
Looking ahead, the team plans to try to generate effective drugs that can target EGFR, AXL, and YAP. "We think that intrinsic YAP activation or acquired re-activation after EGFR-targeted therapy in head and neck squamous cell carcinoma and lung adenocarcinoma has not been clarified yet. We will continue the research," said J. Silvio Gutkind, distinguished professor, Department of Pharmacology, University of California, San Diego.
The research team includes Kento Okamoto, Souichi Yanamoto, and Mutsumi Miyauchi from the Graduate School of Biomedical and Health Sciences, Hiroshima University; Toshinori Ando, Tomoaki Shintani, and Mikihito Kajiya from Hiroshima University Hospital; Hiroki Izumi from National Cancer Center Hospital East, Kashiwa, Japan; Susumu S. Kobayashi from National Cancer Center, Kashiwa, Japan, and Harvard Medical School; and J. Silvio Gutkind from Moores Cancer Center and Department of Pharmacology, University of California, San Diego.
The research is funded by Japan Science and Technology Agency (JST) SPRING, Hiroshima University Foundation, and JST HIRAKU-Global program.
About the study
Journal: Oncogene*
Title: AXL activates YAP through the EGFR-LATS1/2 axis and confers resistance to EGFR-targeted drugs in head and neck squamous cell carcinoma
Authors: Kento Okamoto, Toshinori Ando, Hiroki Izumi, Susumu S. Kobayashi, Tomoaki Shintani, J. Silvio Gutkind, Souichi Yanamoto, Mutsumi Miyauchi & Mikihito Kajiya
DOI: 10.1038/s41388-023-02810-7
*Rank by Journal Citation Indicator (JCI): Q1






