Scientists of the Department of Chemistry of Tomsk State University came up with the fundamentals for the creation of new catalysts with monoatomic and cluster structures. The special structure of the material makes it possible to reduce the amount of active expensive components considerably - palladium, gold, platinum, iridium, thus ensuring its higher activity. The laboratory samples demonstrated high efficiency in the reduction reaction of highly toxic substances - nitroaromatic compounds at room temperature and atmospheric pressure. The results of the study were published in the ACS Applied Nano Materials (Q1). The project is supported by the Russian Science Foundation.
- The whole world is striving to create new nanomaterials with enhanced functional properties. In the field of catalytic materials, a stable scientific trend is the development of single-atom catalysts. Their active component is contained not in the form of nanoparticles, but in the form of individual atoms (single-atom), says project leader Grigory Mamontov, head of the research laboratory of porous materials and sorption at the TSU Faculty of Chemistry. - Within the framework of our project we are developing the fundamentals that will be used to create a line of catalysts to further solve specific environmental problems.
As noted by the scientist, the most active in the oxidation of volatile organic compounds are catalysts based on applied noble metals, primarily palladium and platinum. Their use is limited by their high cost, and the destruction of catalysts in the conditions of catalytic processes leads to irretrievable loss of these expensive metals.
- The task of creating catalysts based on noble metals with high activity, but at the same time with reduced cost can be solved in two ways. The first option is the use of very low contents of metals stabilized in monoatomic and cluster states. For this purpose it is necessary to invent and organize such a structure in which the atoms of the active substance will be stable and provide the material with the necessary catalytic properties, - explains Grigory Mamontov. - The second way is to combine platinum metals with other, more accessible metals, in particular, silver and iron to obtain the corresponding bimetallic structures. This provides a synergistic effect due to the joint action of the two metals. We use both approaches in the development of new catalysts.
The TSU chemists tested their theory in practice. They tested a line of six catalysts based on CeO2 and Fe2O3oxides. Iridium and silver were used as active components.
The catalytic materials were tested in the reduction reaction of nitroaromatic compounds, which are some of the most persistent harmful organic pollutants. Such compounds are found primarily in water used by pharmaceutical, paint and varnish, chemical and other industries.
- Nitrophenols are widely used in industry as intermediates in the synthesis of more valuable compounds - amines. The stable and water-soluble substance - 4-nitrophenol - is the main pollutant of wastewater in the production of medicines, fungicides, insecticides and dyes, adds Grigory Mamontov. - This substance also has a toxic effect on the human body. Thus, the development of methods to neutralize 4-nitrophenol is an important task.
Catalysts convert the harmful substance into amines, which, on the contrary, are useful and can be used in many industries - paints, pharmaceuticals, cosmetics.
Another point of application of the developed fundamentals is the transformation of amines production. Nowadays they are produced under rather "harsh" conditions - at high temperature and pressure, which requires high financial and energy costs. The approaches proposed by TSU chemists correspond to the principles of "green" chemistry and will make it possible to do this under much "softer" conditions - at room temperature and atmospheric pressure.
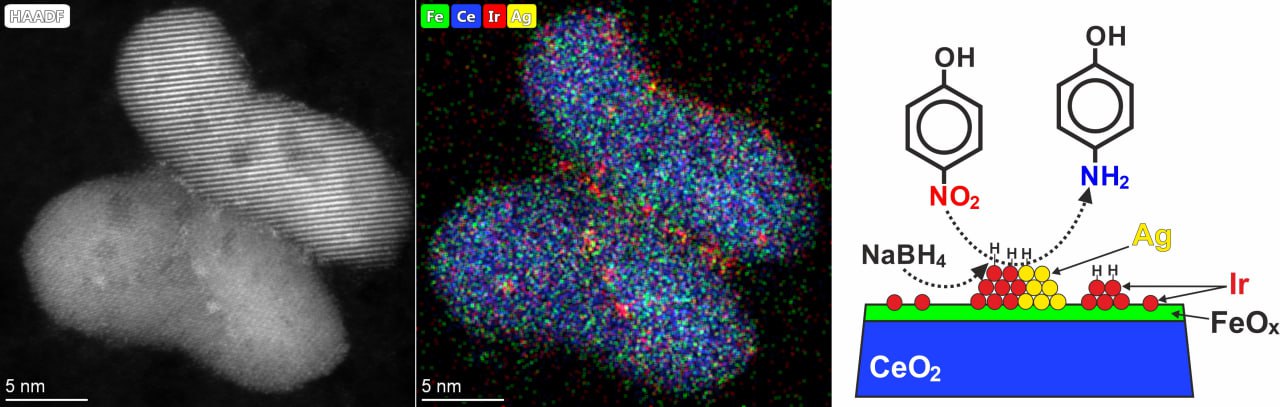
Illustration to the article by TSU scientists
A significant result of the project is that the chemists managed to achieve a synergistic effect due to the special structure and combination of platinum metals with more available metals, in particular iron and silver. For instance, when the amount of active expensive substance, such as iridium, is reduced, the efficiency of the catalyst does not decrease, but on the contrary, increases.
Moreover, the fundamentals for the creation of new catalysts with monoatomic and cluster structures will make it possible to solve practical tasks aimed at ensuring the safety of society and improving the quality of life of people. Neutralization of volatile organic compounds is also of great importance in other strategic areas. For example, an important task is air purification in enclosed or poorly ventilated spaces such as underground parking lots.






