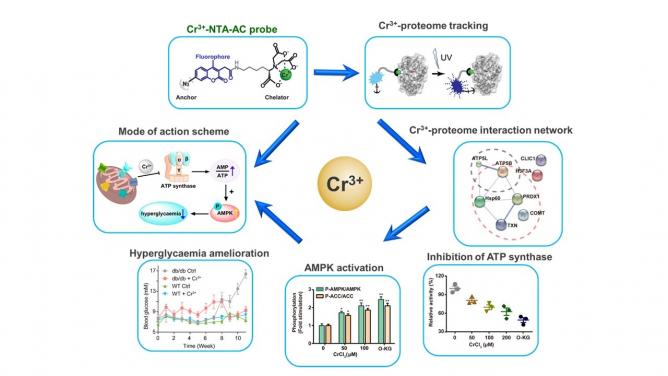
Figure 1. Chromium(Cr3+) fluorescent probes can label Cr(III)-binding proteins specifically in cells (top), Cr(III)-binding proteins are concentrated in the regulation of mitochondrial biological functions (right), and Cr(III) reduces ATP synthase Enzyme activity (bottom right), Cr(III) initiates downstream AMPK pathway (bottom), Cr(III) improves glucose metabolism (bottom left), diagram of Cr(III) mode of action (left).
Recently, a research team led by Professor Hongzhe SUN from the Department of Chemistry, Faculty of Science, The University of Hong Kong, has published a paper in Nature Communications.
The researchers found that, chromium(III) (Cr(III)), a nutritional supplement, can enhance cells' ability to metabolise glucose by regulating ATP synthase activity. This process improves mitochondrial deformation caused by high glucose levels and significantly boosts glucose metabolism in type 2 diabetic mice. To uncover the protein targets of Cr(III) and elucidate the molecular mechanism, the team has developed a fluorescent probe for detecting transient metal-protein interactions, achieving a high spatiotemporal resolution tracking of the Cr(III) proteome in HepG2 cells. This led to the identification of Cr(III)-binding proteins within cells. The team then revealed that Cr(III) replaces magnesium ions (Mg2+) in ATP synthase, reduces ATP synthase activity, and activates the downstream AMPK pathway, resulting in improved glucose metabolism. This study provides a novel concept for hypoglycaemic research.
"Although Cr(III) compounds have long been used as a nutritional supplement for diabetes treatment, weight loss and muscle development, its protein target and mechanism of action remain concealed for over half a century. We used a novel fluorescent probe, along with other chemical biology approaches, to uncover the long-standing scientific problem of the biological chemistry of Cr(III) and discovered that Cr(III) targets ATP synthase to regulate glucose," commented Professor Sun.
Research Background
Diabetes, a chronic metabolic disease with over 500 million patients worldwide, cause nearly 2 million direct deaths each year. Over 95% of diabetic patients suffer from type 2 diabetes mellitus (T2DM), which poses a serious threat to human health. The effect of Cr(III) on anti-type 2 diabetes has been extensively studied in animal models. Since the initial discovery that Cr(III) supplementation in rat diets increased blood sugar removal rates, Cr(III) has been widely used in nutritional supplements for diabetes treatment, weight loss and muscle development. In fact, it is the second best-selling supplement in US, just after calcium mineral.
Despite its significance in food and nutritional science, chromium remains one of the least understood transition metals in the periodic table. For over half a century, the importance, pharmacological properties, and mechanisms of action of Cr(III) in human physiology have been debated. The originally conceived low-molecular-weight chromium(III)-binding peptide (chromodulin) has never been reproducibly identified. Cr(III) is widely believed to improve glucose metabolism, maintain normal blood sugar levels, regulate carbohydrate and lipid metabolism, and enhance insulin signaling. However, the underlying molecular mechanism remains unknown, and the bottleneck in uncovering such a molecular mechanism lies in the difficulty in identifying Cr(III)'s molecular targets in cells. These biomolecules are crucial for elucidating Cr(III)'s physiological and pharmacological effects, constituting the so-called "Holy Grail" of chromium biochemistry.
To date, no proteins that directly bind Cr(III) have been identified in cells or tissues. Various controversial separation and detection methods often produce weak Cr(III) signals, which might be attributed to the dissociation of Cr(III) from its bound proteins. Currently, there is no suitable method for tracking Cr(III)-binding proteins in living cells. For metals that bind weakly or transiently to proteins, labeling metalloproteins with a small molecule fluorescent probe is beneficial for understanding their spatiotemporal distribution and functional regulation in living cells.
Key findings
Building on their previous work, the team synthesised a fluorescent probe for tracking and recognising Cr(III)-binding proteins in living cells. The probe consists of three parts: a fluorophore (coumarin derivative (AC), a metal chelating group (i.e. nitrilotriacetate (NTA)) and a photoactive crosslinking group (i.e. arylazide). Upon chelation with chromium (III), the probe enters the cell, subsequently binds its target proteins, and the arylazide group forms a covalent bond with Cr(III)-binding proteins (i.e. target poteins) upon ultraviolet light irradiation. This leads to fixing the Cr(III)-binding proteins in cells without dissociation during downstream purification.
Using these probes, the team monitored the distribution of Cr(III)-binding proteins in living cells, and found that they were concentrated in the cells' mitochondria. These proteins were identified in conjunction with two-dimensional gel electrophoresis and mass spectrometry. The researchers discovered that Cr(III) binds residues of Thr213 and Glu242 in ATP synthase's active site, replacing magnesium ion (Mg2+), reducing ATP synthase activity, and leading to AMPK activation. The AMPK pathway then alleviates mitochondrial damage caused by high glucose and improves glucose metabolism. This mode of action was successfully verified in a mouse model of type II diabetes. This study addresses how Cr(III) improves hyperglycaemic stress at the molecular level for the first time. With the identification of multiple Cr(III)-binding proteins, this study also opens a new horizon for further investigation of the pharmacological role of Cr(III) in other diseases other than anti-diabetes, such as anti-neurodegenerative diseases and anti-aging new horizons.
The reviewers speak highly of this work "It is an immensely important aspect of basic science and its implications for human health, and it is exactly what is needed: the identification of molecular targets, an endeavour that has met with rather limited success in the last 70 years. Chromium supplements are a huge market worldwide (sold for weight loss, and muscle building). With controversies about their mode of action, it is rather questionable gambling with our health. Thus, the work addresses a major health issue that needs to be carefully evaluated for either the protection of large populations or the benefit for them concerning antidiabetic actions of chromium(III). The authors address chromium biology with a new approach of chemical biology and an impressive number of biochemical, molecular biological, and animal experiments."
About the research team
This study was done jointly by The University of Hong Kong, Eco-Environmental Research Center of the Chinese Academy of Sciences, and City University of Hong Kong. Dr Haibo WANG and Dr Hongyan LI (The University of Hong Kong), and Dr Ligang HU (Eco-Environmental Research Center) are the co-first authors of this paper. Other members of HKU Department of Chemistry participating in the research include Dr Yau-Tsz LAI, postgraduate student Ms Xueying WEI, Dr Xiaohan XU, Dr Zhenkun CAO, and Dr Yuen-Yan CHANG. Professor Aimin XU of HKU Li Ka Shing Faculty of Medicine, Professors Guibin JIANG and Professor Qunfang ZHOU of the Eco-Environmental Research Center of the Chinese Academy of Sciences, Professor Mingliang HE and Dr Qianya WAN of the City University of Hong Kong, and Associated PI, Dr Huiming CAO of Jianghan University. The Research Grants Council of Hong Kong, and the Health Research Fund and the Basic Research Seed Fund of the Food and Health Bureau of the Hong Kong SAR support this study. The research work was supported by the University of Hong Kong (Norman & Cecilia Yip Foundation and URC).
About Professor Hongzhe Sun
Professor Hongzhe SUN is the Norman & Cecilia Yip Professor in Bioinorganic Chemistry and Chair Professor of Chemistry at The University of Hong Kong. His research lies in metalloproteomics and metallomics, discovery of antimicrobial and antiviral agents, and inorganic chemical biology.
To view the research paper "Mitochondrial ATP synthase as a direct molecular target of chromium(III) (Cr(III)) to ameliorate hyperglycaemia stress", please visit: https://www.nature.com/articles/s41467-023-37351-w
Image download and description: https://www.scifac.hku.hk/press






