Details of a cellular adaptation mechanism may point to new ways of treating neurodegeneration
Stress management is a crucial skill - not only for us, but for our cells too. When cells face extremely stressful conditions, they produce tiny, temporary clumps called stress granules that help them cope with heat, cold or other potentially harmful circumstances. If these granules are not dismantled after the stress subsides, they can become scrambled, cluttering the cell like wrecked cars filling up a junkyard, and this cluttering, scientists believe, may lead to neurodegenerative disease. Researchers at the Weizmann Institute of Science, together with collaborators, have deciphered the molecular mechanism by which stress granules are normally dismantled, and they have shown that manipulating this mechanism can block neurodegeneration of the type seen in amyotrophic lateral sclerosis, or ALS.
Stress granules consist of messenger RNA molecules and assorted proteins that bind to the RNAs. How they are assembled has been known for a while, but how they undergo the reverse, disassembly process was unknown. Prof. Eran Hornstein of Weizmann's Molecular Genetics Department and his team set out to study this reverse process in order to understand what happens when it goes awry. Aberrant stress granules that fail to disassemble are thought to be precursors of abnormal protein aggregates, called inclusions, found in the brains of people with ALS and a number of other neurodegenerative diseases, among them a common type of dementia known as frontotemporal degeneration.

Hagai Marmor-Kollet and Aviad Siany, graduate students in Hornstein's lab, led the study, which was conducted in collaboration with Prof. Tamar Geiger of Tel Aviv University and researchers from Harvard Medical School and KU Leuven. The scientists developed new methods that make it possible to isolate even the tiniest compartments from human cells, labeling the proteins within them so that they can later be identified using mass spectroscopy. With this method, the researchers defined the composition of stress granules, revealing previously unknown components, including more than 100 new proteins and microscopic substructures. Continuing to investigate, they were surprised to see that as the disassembly progressed, dozens of additional proteins were recruited to the granules. The scientists called these "disassembly-engaged proteins" - proteins that are involved in the dismantling.
When the scientists focused on how, exactly, the disassembly-engaged proteins dissolve the granules, they identified a previously unknown pathway of biochemical reactions that presented them with yet another surprise. It turned out that key elements of this pathway are protein tags called SUMO (small ubiquitin-like modifiers), which were thought to perform entirely different functions that take place in the cell nucleus.
If these granules are not dismantled after the stress subsides, they can become scrambled, cluttering the cell like wrecked cars filling up a junkyard
The scientists then performed a series of experiments showing that their findings are relevant to human neurodegenerative diseases. After exposing cells to a toxic protein known to cause ALS in humans, they found that stress granules failed to be dismantled properly in these cells, and the disassembly-engaged proteins, particularly the SUMO enzymes, failed to be properly recruited. Most importantly, the scientists managed to block neurodegeneration in fruit flies that had been genetically engineered to develop a disease similar to ALS, by introducing the SUMO enzymes into their degenerating bodies.
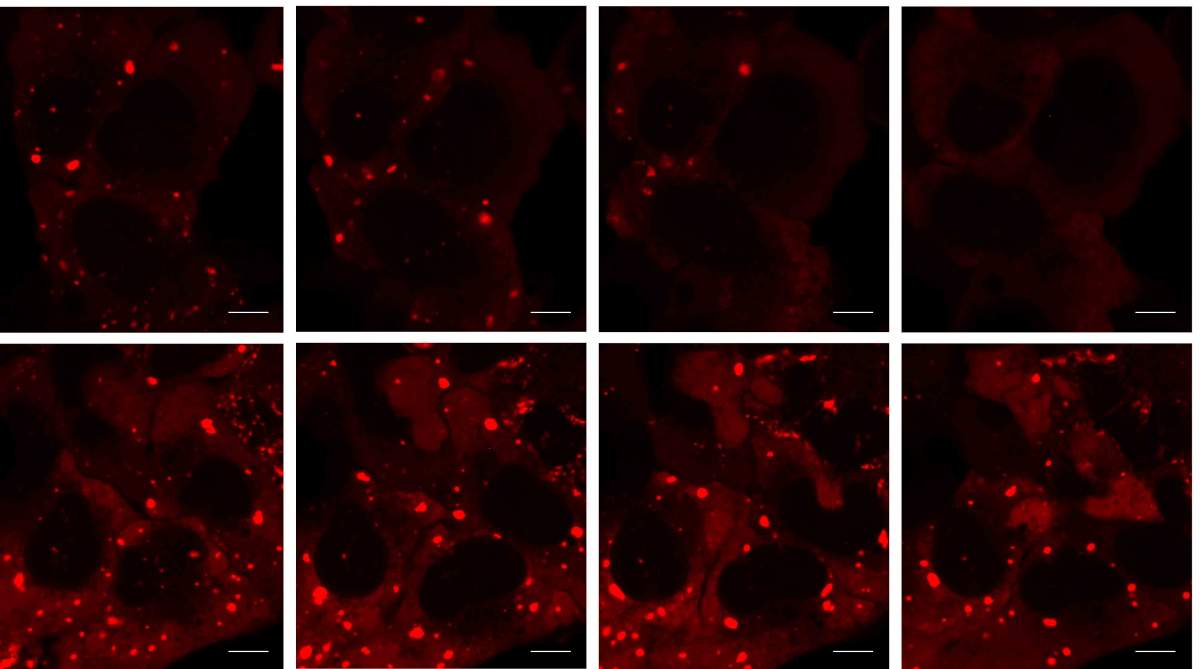
These findings open up a new line of research into the mechanisms underlying ALS and other neurodegenerative diseases: clarifying how defects in a normal adaptive mechanism, the formation and breakdown of stress granules, can contribute to these disorders.
"The study will hopefully serve as the basis for developing potential future therapies," says Hornstein. "Our findings point to a promising new way of battling neurodegeneration - by enhancing the activity of SUMO enzymes."






