Clinical trials to continue the development of a liver dialysis device to treat those with life-threatening liver disease have been funded by a grant awarded to UCL, the Royal Free Hospital and Yaqrit.
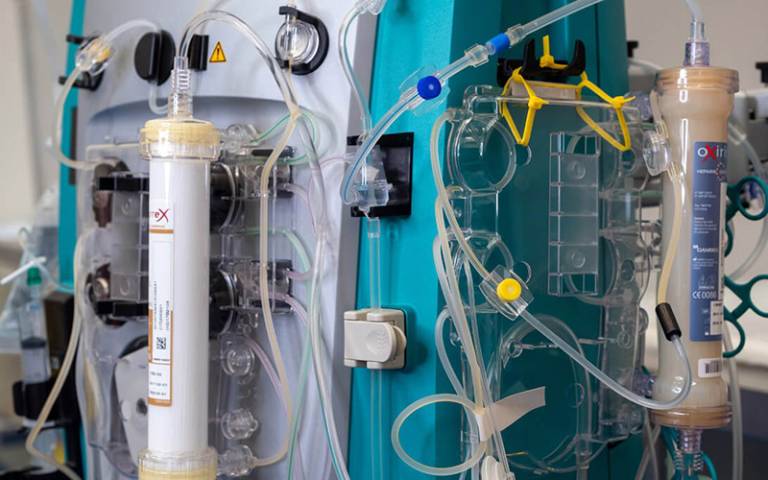
The £2.2 million grant, awarded by the National Institute of Health and Care Research (NIHR), covers the clinical and development costs of clinical trials of the DIALIVE device (also called YAQ002), which was developed at UCL and is licensed to UCLB spinout Yaqrit.
DIALIVE uses dialysis, a technique to clean the blood of harmful substances more familiar in the treatment of those with serious kidney disease, to treat patients with severe Acute-on-Chronic Liver Failure (ACLF).
ACLF is a life-threatening condition affecting patients with cirrhosis (liver scarring) that leads to multiple organ failure and carries a high risk of mortality. Globally, it affects up to 3.7 million patients with liver cirrhosis each year, including around 1.9 million with multiple organ failure. Depending on disease severity, around 40-80% of patients die within 28 days.
The device has already proved to be a safe and rapid way of resolving liver failure by allowing liver regeneration to occur. In a successful earlier Europe-wide study led by UCL, ACLF was resolved approximately twice as frequently and much more rapidly during the 10-day treatment period in DIALIVE-treated patients compared to those on standard-of-care treatments.
Troels Jordansen, Chief Executive Officer of Yaqrit, said: "The NIHR grant financing for YAQ002 enables Yaqrit to take this life-saving technology forward towards the market, as part of our mission to create value by helping patients with liver failure. The prestige and resources of NIHR will accelerate Yaqrit's development of YAQ002 bringing new hope to thousands of advanced liver patients who desperately need an effective treatment for ACLF."
Results of the trial are expected in 2027, following formal ethical clearance and a patient recruitment period of around 18 months. The trial will recruit around 70 of the sickest patients who have a high risk of dying unless they receive a liver transplant.
Following enrolment, during a 10-day treatment period, those patients randomised to be supported by DIALIVE will receive up to seven sessions of treatment.
Professor Rajiv Jalan, co-lead investigator of the trial from UCL Institute for Liver & Digestive Health and founder of Yaqrit, said: "This is extremely important news for patients within the UK and beyond with advanced liver disease whose current treatment options are severely limited. Our goal is to demonstrate that we can resolve ACLF more often and/or faster than standard of care, and thereby impact both patients' time in hospital and chances of survival."
Leading the study as co-principal investigators are Professor Banwari Agarwal (Royal Free Hospital and UCL Division of Medicine) and Dr Rohit Saha (Royal Free Hospital and King's College London). Twelve other UK clinical centres specialising in the treatment of ACLF will also be involved in the recruitment of patients. Other collaborators include Dr Sameer Patel of King's College Hospital London and Dr Mansoor Bangash of the Queen Elizabeth Hospital, Birmingham.
UCL and Royal Free Hospital researchers identified acute-on-chronic liver failure as a distinct clinical entity in 2001. This work led to DIALIVE, which was invented by Professor Jalan and Professor Nathan Davies, based in the Liver Failure Group at UCL's Institute for Liver and Digestive Health, part of UCL Division of Medicine. The intellectual property behind the technology was patented by UCL in 2009 and licensed to a spin-out company, Yaqrit.






