A research project at EPFL succeeded in decoding the complex dance of electrons in water, a major step in understanding a critical process of many chemical phenomena, and that might be the first step to improving energy conversion technologies.
Water, the cradle of life on Earth, is not just a passive backdrop but actively participates in the chemical ballet of life. Central to this dance is the behavior of electrons, particularly during a process known as charge transfer to solvent (CTTS).
CTTS is like a microscopic dance where an electron from something dissolved in water, like salt, leaps out and joins the water itself. The process creates a now "hydrated" electron, which is a key element of many aqueous reactions, like the ones underlying life itself. Consequently, CTTS is essential for understanding how electrons move in solutions.
In a new EPFL study, researchers Jinggang Lan, Majed Chergui, and Alfredo Pasquarello have studied the intricate interactions between electrons and their solvent environments. The work was conceived and primarily carried out at EPFL, with finalizing contributions from Jinggang Lan upon him taking on a postdoctoral fellowship in the Simons Center for Computational Physical Chemistry at New York University.
Looking at the CTTS process, the researchers meticulously visualized the dynamic interplay between the escaping electron and the polarizing water molecules surrounding it, marking a significant leap in our comprehension of such complex interactions.
The team used iodide dissolved in water ("aqueous iodide"), because it makes it easier to understand how electrons move to the surrounding water. Iodide, like table salt, doesn't have complex internal movements, which makes it simpler to study. This allowed the scientists to observe how iodide can swiftly release an electron into the surrounding water, a process influenced by the arrangement of water molecules around the iodide.
To study the CTTS process, the researchers used ab initio molecular dynamics, a sophisticated technique that simulates the behavior of molecules in a computer by calculating atomic interactions and movements from fundamental physical principles using quantum mechanics. "Ab initio" means "from the beginning" in Latin, indicating that this method starts from fundamental physical principles, allowing scientists to accurately predict how molecules and materials evolve over time without relying on empirical data for the interactions between particles.
Combining the ab initio approach with sophisticated machine learning techniques, the scientists were able to visualize and analyze the CTTS process in unprecedented detail, tracking the journey of an electron from being attached to an iodide ion to becoming solvated - being surrounded and stabilized by water molecules.
The study revealed that CTTS involves a series of distinct states, each characterized by the distance between the electron and the iodine nucleus: from being closely associated with the iodine atom (contact-pair state), to separating into the solvent (solvent-separated state), and finally becoming fully solvated as a hydrated electron.
"The advance mostly rests at the fundamental level," says Alfredo Pasquarello. "The described mechanism involves a subtle interplay between electronic excitation and ionic polarization effects, which produce a sequence of configurations as revealed by our simulations."
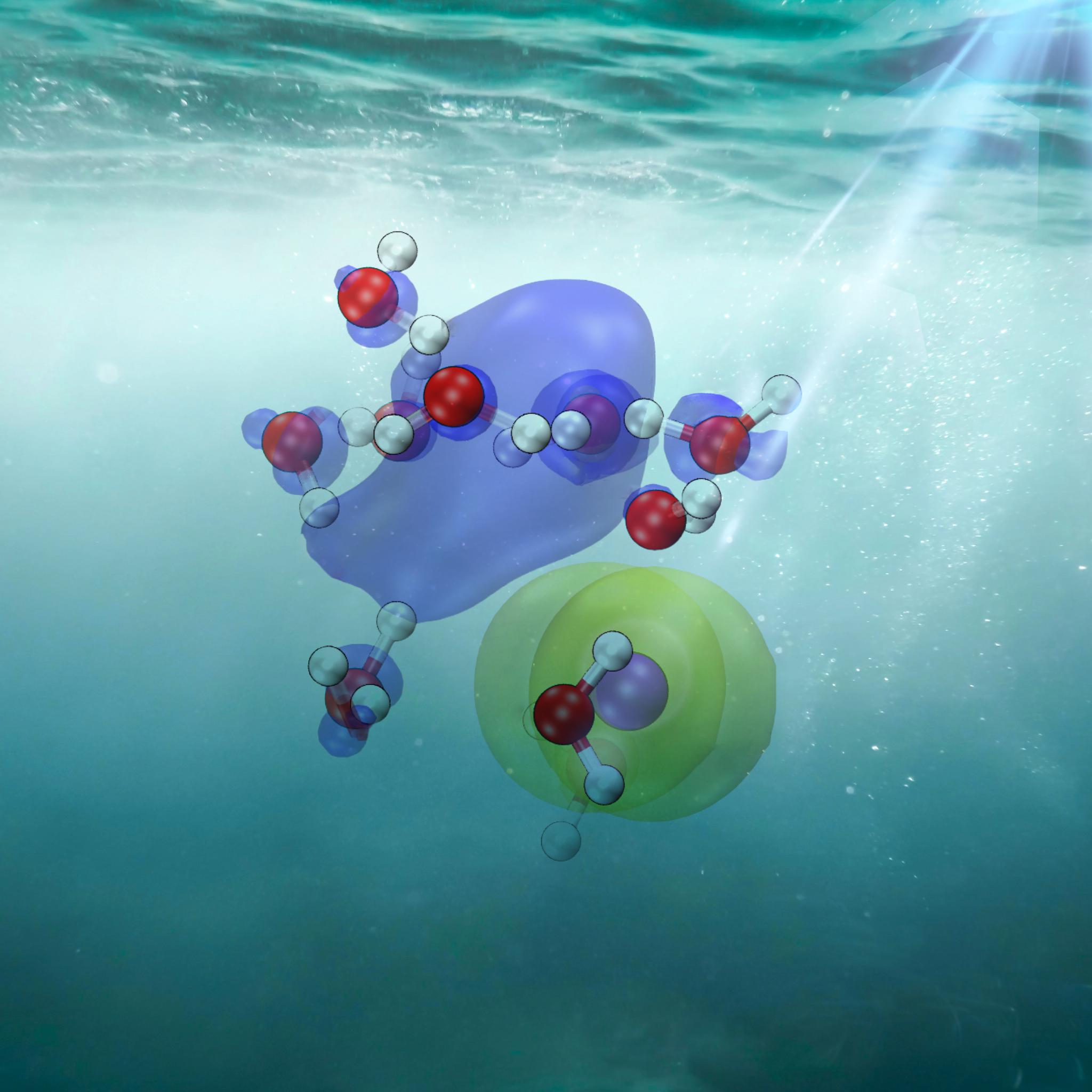
But shedding light on CTTS could also have implications into a wide range of applications involving charge and energy transfer reactions. Understanding how electrons interact with their environment at such a fundamental level could be key to developing more efficient solar energy conversion systems, improving photocatalysis techniques, and even advancing our knowledge of material science and environmental processes.
"Understanding charge transfer to solvent provides insights into the behavior of energy and electrons in chemical reactions, influencing a range from natural biological activities to the technology used in energy conversion," says Jinggang Lan.






