Microalgae-which are major contributors to global photosynthesis and primary productivity-serve as promising chassis cells in synthetic biology.
In a study published in Plant Communications, researchers from the Qingdao Institute of Bioenergy and Bioprocess Technology of the Chinese Academy of Sciences have unraveled the distribution pattern and dynamic changes in DNA N6-methyladenine (6mA) at a single-base resolution in wild-type and 6mA-disrupted mutant strains, thus revealing its critical role in lipid accumulation, especially under high light conditions.
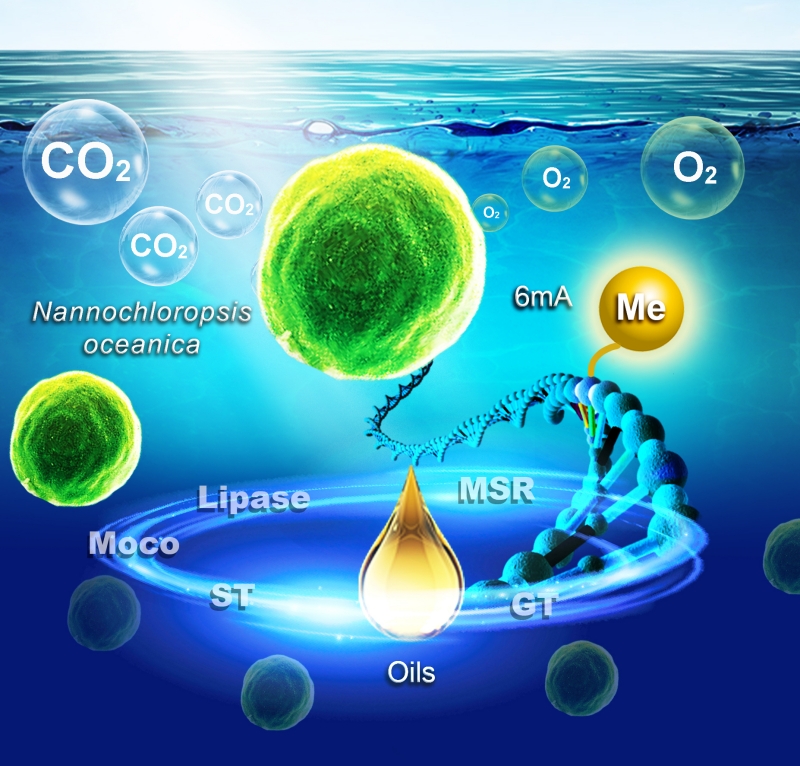
Nannochloropsis oceanica, known for its robustness and efficiency in outdoor cultivation, offers advantages such as rapid growth, strong tolerance for carbon dioxide, robust lipid synthesis, and high quality unsaturated fatty acids. With a small genome size (~30 Mb) and haploid nature, it allows for flexible genetic manipulation, including gene knockout, overexpression, large genomic fragment deletion, and homologous recombination, with high editing efficiency.
6mA is an important DNA methylation modification. Using single-molecule real-time sequencing, the researchers revealed the whole-genome 6mA landscape of Nannochloropsis oceanica. The results highlight the preferential enrichment of 6mA in the AGGYV motif, elevated levels within transposons and 3' untranslated regions, and a close association with active transcription.
"We observed a gradual increase of 6mA along the gene transcription direction, with specific enrichment near splice donors and transcription termination sites," said GONG Yanhai, co-first author of the study.
In addition, highly expressed genes show a higher abundance of 6mA in the genome compared to lowly expressed genes, suggesting a positive interplay between 6mA and general transcription factors.
To further investigate the effects of 6mA, the researchers knocked out the 6mA methyltransferase gene (NO08G00280). This resulted in altered methylation patterns and changes in the expression of key genes associated with the molybdenum cofactor, sulfate transporters, glycosyl transferase, lipase, and methionine sulphoxide reductase, ultimately leading to a decrease in biomass and oil production.
Conversely, knockout of the demethylase gene (NO06G02500) resulted in increased 6mA levels and slowed growth.
"These findings not only validated key enzymes in the epigenetic regulation pathway, but also shed light on the pivotal role of 6mA in lipid accumulation in Nannochloropsis under high light conditions," said Prof. WANG Qintao, co-first author of the study.
These findings provide insights into the use of epigenetic genome modifications to increase biomass and lipid production efficiency in industrial microalgae. The study is part of the Nannochloropsis Design & Synthesis Initiative (NanDeSyn), which involves 26 research teams from eight countries, coordinating efforts to advance molecular breeding and synthetic biology research in industrial carbon-fixing, oil-producing microalgae.
"Through our collaborative efforts, we've freely shared comprehensive datasets that include whole-genome 6mA epigenetic modification maps, transcriptomes, and associated mutants under various cultivation conditions. These valuable resources are now available to the scientific community through the NanDeSyn website. Our common goal is to advance industrial microalgae research by promoting the exchange of germplasm resources, genetic tools, and functional genomics information," said Prof. XU Jian, corresponding author of the study.