Can copper be turned into gold? For centuries, alchemists pursued this dream, unaware that such a transformation requires a nuclear reaction. In contrast, graphite-the material found in pencil tips-and diamond are both composed entirely of carbon atoms; the key difference lies in how these atoms are arranged. Converting graphite into diamond requires extreme temperatures and pressures to break and reform chemical bonds, making the process impractical.
A more feasible transformation, according to Prof. Moshe Ben Shalom, head of the Quantum Layered Matter Group at Tel Aviv University, involves reconfiguring the atomic layers of graphite by shifting them against relatively weak van der Waals forces. This study, led by Prof. Ben Shalom and PhD students Maayan Vizner Stern and Simon Salleh Atri, all from the School of Physics & Astronomy at Tel Aviv University, was recently published in the prestigious journal Nature Review Physics.

The research team
While this method won't create diamonds, if the switching process is fast and efficient enough, it could serve as a tiny electronic memory unit. In this case, the value of these newly engineered "polytype" materials could surpass that of both diamonds and gold.
Like LEGO, But Atomic
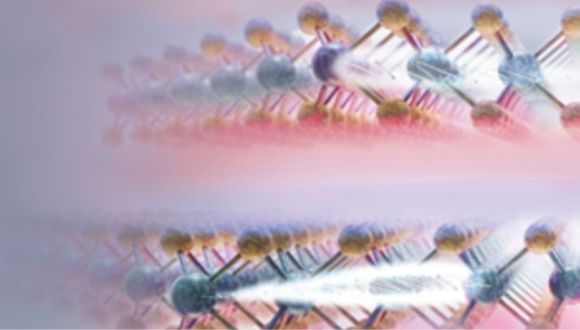
PhD student Maayan Vizner Stern explains: "Like graphite, nature produces many other materials with weakly bonded layers. Each layer behaves like a LEGO brick-breaking a single brick is difficult, but separating and reconnecting two bricks is relatively simple. Similarly, in layered materials, the layers prefer specific stacking positions where atoms align perfectly with those in the neighboring layer. Sliding between these positions happens in tiny, discrete jumps-just an atomic distance at a time".
PhD student Simon Salleh Atri describes their research: "We are developing new methods to slide the layers into different arrangements and study the resulting materials. By applying an electric field or mechanical pressure, we can shift the layers into various stable configurations. Since these layers remain in their final position even after the external force is removed, they can store information-functioning as a tiny memory unit".
A New World of Materials Emerges, Layer by Layer
Their team has also explored how different numbers of layers influence material properties. For example, three layers of a material with two types of atoms can create six distinct stable materials, each with unique internal polarizations. With five layers, this number increases to 45 different possible structures. By switching between these configurations, researchers can control electrical, magnetic, and optical properties. Even graphite, composed solely of carbon, can rearrange into six different crystalline forms, each with distinct electrical conductivities, infrared responses, magnetizations, and superconducting properties.
The main challenge is maintaining the material's stability while ensuring controlled structural transitions. Their recent perspective paper summarizes ongoing studies and proposes new methods to refine this "Slidetronics" switching mechanism, paving the way for innovative applications in electronics, computing, and beyond.
With continued research, these sliding materials could revolutionize technology, offering faster, more efficient memory storage and unprecedented control over material properties. The ability to manipulate atomic layers with precision is opening doors to a new era in material science-one where the most valuable discoveries may not come from creating gold, but from unlocking the hidden potential of everyday elements.






