A new technology developed by the Single-Cell Center at Qingdao Institute of Bioenergy and Bioprocess Technology (QIBEBT) of the Chinese Academy of Sciences, in collaboration with several institutions, is poised to transform the discovery of microbial species and enzymes with specific in situ metabolic functions.
The team's findings were published in The Innovation.
The new technology, FISH-scRACS-seq (Fluorescence In Situ Hybridization-guided Single-Cell Raman-activated Sorting and Sequencing), utilizes a Raman-activated optical tweezers-based cell sorter developed by Qingdao Single-Cell Biotech Inc. This innovative method combines species-targeting fluorescence in situ hybridization (FISH) with Raman spectroscopy, allowing for the direct identification and isolation-from environmental samples-of functional single cells and the enzymes they encode.
This dual-targeting approach allows researchers to align specific microbial species with their respective metabolic functions, revealing the genomic resources that drive these processes. It facilitates a high-resolution examination of ecological interactions, connecting species, their metabolic capabilities, genome sequences, metabolic pathways, and enzyme activities.
"FISH-scRACS-seq technology opens up new possibilities for studying microbial metabolic functions in natural environments," said Associate Prof. JING Xiaoyan, first author of the study, from the Single-Cell Center at QIBEBT.
"By addressing the limitations of existing single-cell omics approaches-which are either gene-driven (and therefore miss functional aspects) or metabolism-driven (and unable to target specific organisms)-FISH-scRACS-seq allows for high-resolution analysis of microbial functions, metabolic pathways, and genome sequences directly within natural ecosystems," said Prof. XU Jian, the director of Single-Cell Center who heads this work.
The research team utilized this technique to identify the cells, pathways, and enzymes from γ-proteobacteria that are actively involved in degrading cycloalkanes in marine environments. Their analysis uncovered a previously unknown enzyme system, referred to as P450, that is responsible for the degradation of cycloalkanes. This discovery is crucial for bioremediation efforts in aquatic ecosystems contaminated by hydrocarbons.
In experiments conducted with complex soil and mixed-culture systems, FISH-scRACS-seq demonstrated the ability to achieve over 99% single-cell genome coverage. This high coverage enables thorough investigation of enzymatic genes and regulatory elements that facilitate the metabolic functions of host cells in their natural environment.
A key finding of the study was the identification of Pseudoalteromonas fuliginea, a bacterium that can effectively degrade cyclohexane through a newly discovered cytochrome enzyme system, referred to as P450PsFu.
This enzyme system transforms the toxic compound cyclohexane into the non-toxic compound cyclohexanol. This process marks the initial step in the degradation pathway and provides valuable insights into the dynamics of the marine carbon cycle. Interestingly, P450PsFu is uncommon in marine microorganisms. However, its widespread presence in the ocean, especially in cold marine ecosystems, creates new opportunities for understanding hydrocarbon biodegradation in marine environments.
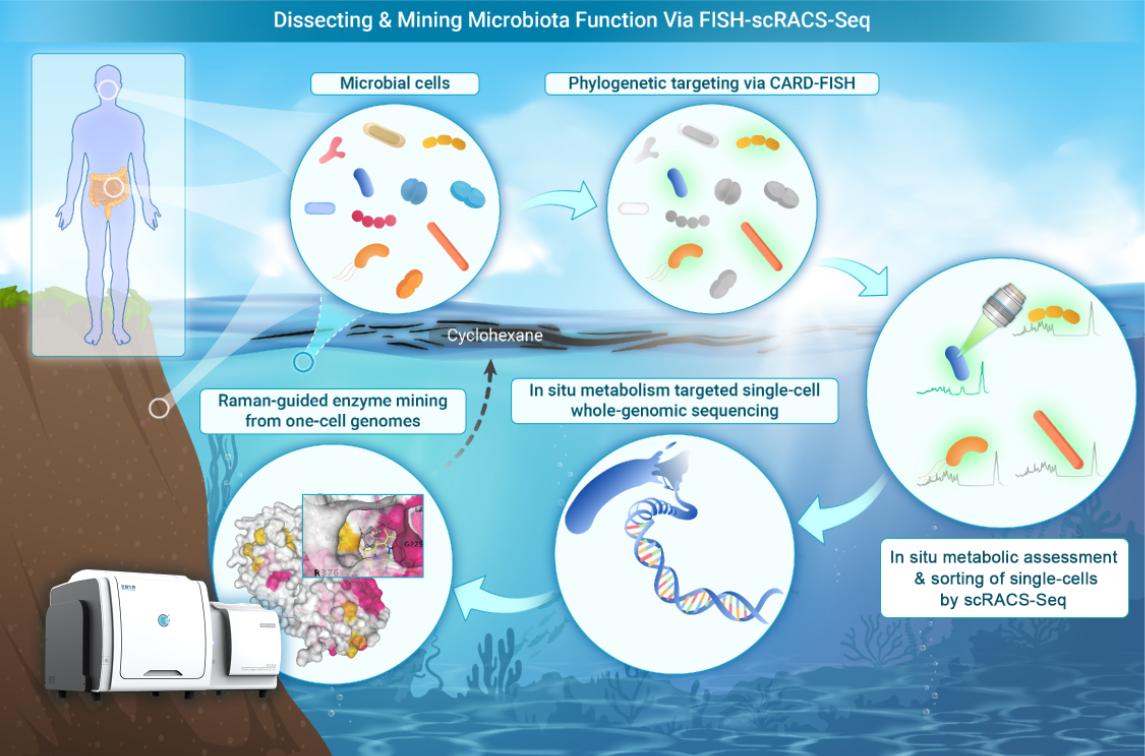
Phylogeny-metabolism dual-directed cell analysis, sorting, and sequencing (Image by LIU Yang)






