Allowing the direct observation of biomolecules in dynamic action, high-speed AFM has opened a new avenue to dynamic structural biology. An enormous amount of successful applications within the last 15 years provide unique insights into essential biological processes at the nanoscale - visualizing, for example, how molecular motors execute their specific functions.
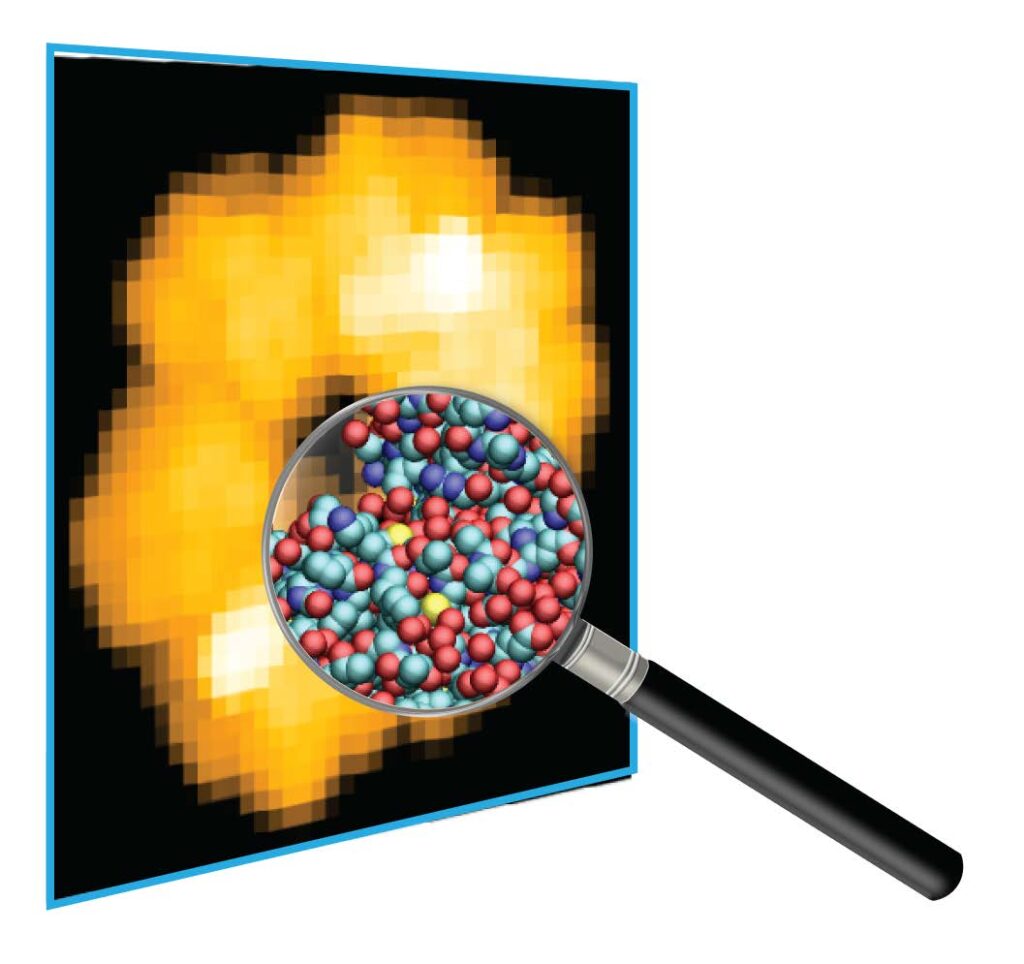
An intrinsic limitation in AFM imaging is that only the surface topography can be acquired, and the AFM tip is too large to resolve details below the nanometer scale. To facilitate the interpretation and understanding of HS-AFM observations, post-experimental analysis and computational methods play an increasingly important role.
In their review paper published in the Current Opinion in Structural Biology journal Holger Flechsig (NanoLSI, Computational Science), and Toshio Ando (Distinguished Professor at NanoLSI) provide an overview of developments in this topical field of interdisciplinary research. Computational modeling and simulations already allow to reconstruct 3D conformations with atomistic resolution from topographic resolution-limited AFM images. Furthermore, quantitative analysis methods allow for example automated recognition of biomolecular shape changes from topographic images, or feature assignment including the identification of amino acid residues on the molecular surface.
The developed computational methods are often implemented in open-access software, allowing for convenient applications by the broad Bio-AFM community to complement experimental observations. In that regard, the BioAFMviewer software project initiated at Kanazawa university in 2020 has gained significant attention and plays an important role in a plethora of collaboration projects.
Combining high-speed AFM and computational modeling will elevate the understanding of how proteins function in atomistic detail. An ambitious future goal is the application of molecular modeling to reconstruct atomistic-level 3D molecular movies from high-speed AFM topographic movies.
Computational modeling allows to infer atomistic information from AFM images
Reference
Holger Flechsig and Toshio Ando. Protein dynamics by the combination of high-speed AFM and computational modeling. Current Opinion in Structural Biology. 80, 102519. 2023.
DOI:10.1016/j.sbi.2023.102591
URL: https://doi.org/10.1016/j.sbi.2023.102591