Catalysts are the keys to speeding up sluggish reactions that require large amounts of energy. Therefore, analyzing which types of catalysts speed up reactions the most and what their underlying mechanisms are can help us improve efficiency. A research team at Tohoku University's Advanced Institute for Materials Research (AIMR) has proposed a strategy to use spinel oxides to improve a reaction called the oxygen evolution reaction (OER). This catalyst overcomes issues typically encountered with previous spinel oxides by involving a rare-earth cerium (Ce) substitution.
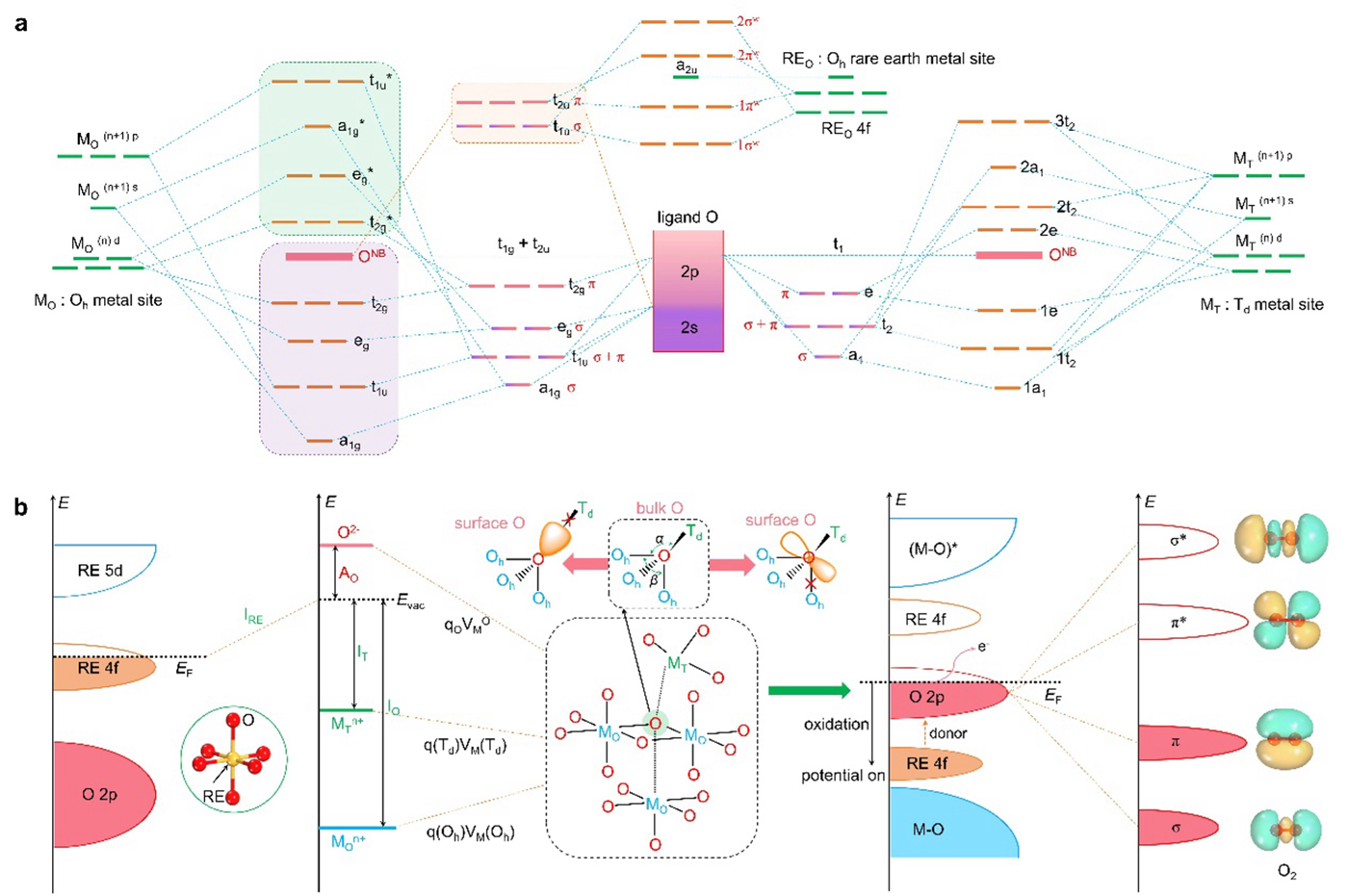
Spinels refer to minerals such as magnesium, nickel, or zinc packed tightly into cubic crystals. Spinel oxides have been explored for their potential as active electrochemical oxygen evolution reaction catalysts. However, the catalytic process for spinel oxides often follows the adsorbate evolution mechanism pathway, which largely inhibits their ability to release O2. This is not helpful for the desired oxygen evolution reaction.
Comparatively, the lattice oxygen mechanism pathway (where the release of O2 is assisted by lattice oxygen from oxides) is much more efficient. In order to best utilize this method, it is important to understand factors such as how to trigger lattice oxygen activation during the oxygen evolution process.
"Even the slightest change can alter how efficient the reaction is," explains Associate Professor Hao Li of AIMR, "Essentially, we want to reliably be able to control which pathway the spinel oxide tends towards, so we can reach the desired outcome every time."
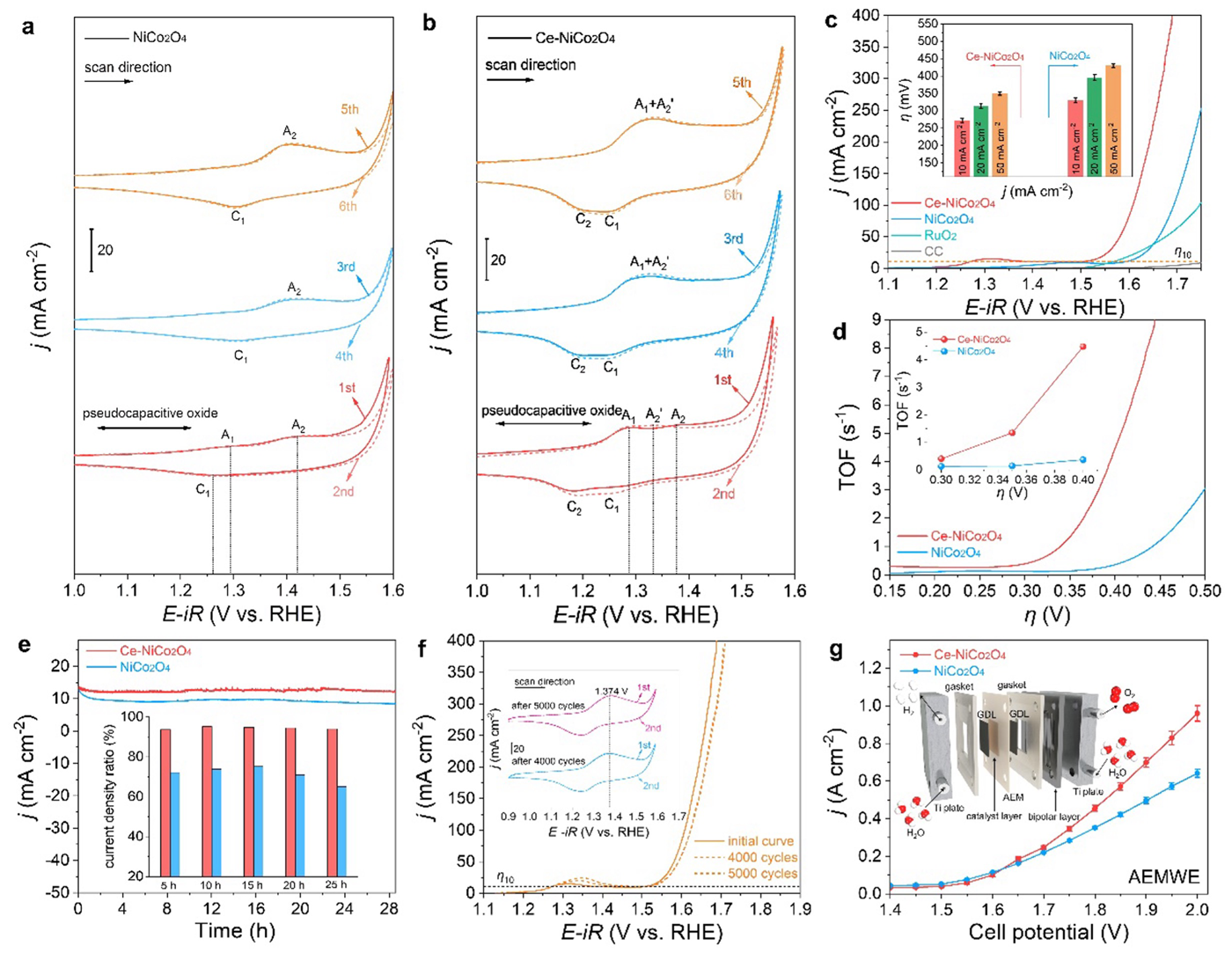
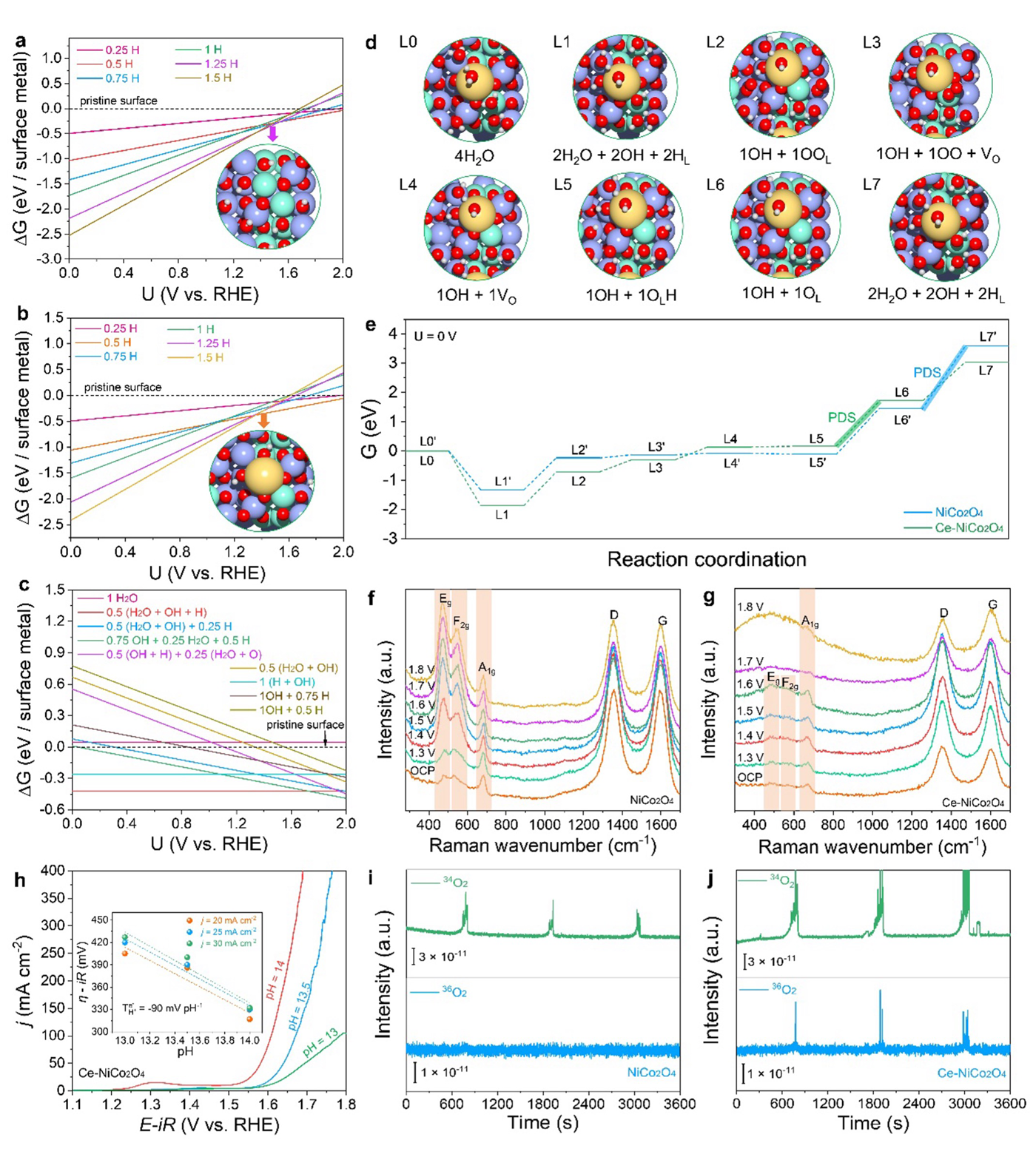
The research team used NiCo2O4 as a model with Ce. The catalyst exhibited remarkable OER activity with a low overpotential, satisfactory electrochemical stability, and good practicability in anion-exchange membrane water electrolyzer. Theoretical analyses reveal that OER on the Ce-NiCo2O4 surface follows the more favorable lattice oxygen mechanism (LOM) pathway compared to NiCo2O4, as further verified by pH-dependent behavior and in situ Raman analysis. In order to better characterize the ins-and-outs of the reaction, electrochemical mass spectrometry was used to confirm that the oxygen was specifically originating from the lattice oxygen of Ce-NiCo2O4.
The addition of Ce was successful in promoting the lattice oxygen pathway, which opens new doors for using this and similar catalysts in electrochemical reactions. This work provides a new perspective for designing highly active spinel oxides for OER and offers significant insights into the rare-earth-enhanced LOM mechanism.
In particular, the OER water-splitting process is useful for producing green hydrogen fuels, hence the intense interest in this reaction. In order for things to change so that these environmentally-friendly fuels can start being used more widely, it all starts with a catalyst.
These findings were published in Angewandte Chemie International Edition on October 9, 2024.
- Publication Details:
Title: Importing Atomic Rare-Earth Sites to Activate Lattice Oxygen of Spinel Oxides for Electrocatalytic Oxygen Evolution
Authors: Xuan Wang, Jinrui Hu, Tingyu Lu, Huiyu Wang, Dongmei Sun, Yawen Tang, Hao Li, Gengtao Fu
Journal: Angewandte Chemie International Edition






