A UC Davis Health team today announced the launch of the world's first, FDA-approved human clinical trial using stem cells before birth to treat the most serious form of spina bifida.
 Researchers at UC Davis Health have developed a special stem cell patch for spina bifida that may help more children with spina bifida walk.
Researchers at UC Davis Health have developed a special stem cell patch for spina bifida that may help more children with spina bifida walk.
The condition is a birth defect that occurs when the spine and spinal cord don't form properly. Without treatment, an exposed spinal cord causes severe neurological damage, resulting in problems that can include lifelong cognitive, mobility, urinary and bowel disabilities.
The one-of-a-kind treatment will be delivered while the baby is still in the mother's womb (in utero). It will be the standard surgical procedure combined with the use of a unique stem cell "patch" to repair the defect before birth.
The team anticipates seeing improvements for those born with the most severe form of spina bifida known as myelomeningocele (MMC).
"Currently, the standard of care for our patients is fetal surgery, which, while promising, still leaves more than half of children with spina bifida unable to walk independently," said Diana Farmer, professor and chair of surgery at UC Davis Health and principal investigator on the study. "There is an extraordinary need for a treatment that prevents or lessens the severity of this devastating condition. Our team has spent more than a decade working up to this point of being able to test such a promising therapy."
In the United States, four babies a day are born with spina bifida (about 1 in every 2,700 live births each year). The condition leaves a portion of the spinal cord and nerves exposed without any bone or skin covering them. It causes a variety of problems because the spinal cord controls a person's ability to move their legs and walk. It can also lead to extra fluid in and around the brain (hydrocephalus), causing brain injury.
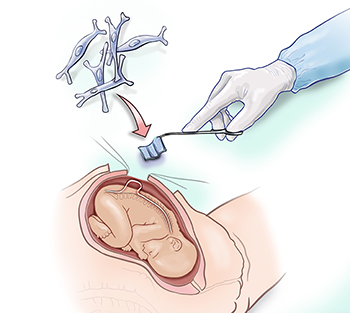
Stem cells are placed on the spinal cord using a special patch during fetal surgery (before birth) to repair the spina bifida defect.
Farmer, stem cell bioengineer Aijun Wang, and the clinical fetal treatment team led by Shinjiro Hirose, plan to treat six patients. During their procedure, the fetal surgeon will place a biological scaffold of special stem cells (in this case, placental mesenchymal stem cells) directly over the exposed spinal cord. The surgeon will then close the opening in the baby's back to allow the tissue to regenerate and protect the infant's spinal cord.
The clinical trial, known formally as the "CuRe Trial: Cellular Therapy for In Utero Repair of Myelomeningocele," is funded by the state's stem cell agency, CIRM. Working with the UC Davis School of Veterinary Medicine, the team tested the stem cell patch and surgical repair in animals with the MMC defect. They found that it enabled the animals born with spina bifida to walk. It also showed that the stem cell treatment was safe in animals.
"We anticipate being able to safely and successfully repair the birth defect that occurs when the protective tissue around a baby's developing spinal cord fails to fully close before birth," said Wang, an associate professor of surgery and biomedical engineering, and co-director of UC Davis' surgical bioengineering laboratory. "Our cellular therapy approach, in combination with surgery, should encourage tissue regeneration and help patients avoid devastating impairments throughout their lives."
Farmer and Wang's team has generated the stem cells for the study from placental tissue. The cells are known to be among the most promising type in regenerative medicine. They have been produced and screened in a highly specialized facility within UC Davis' Institute for Regenerative Cures in Sacramento.
"We've been preparing for this important clinical trial for many years," said Jan Nolta, director of the UC Davis Stem Cell Program. "We often call these mesenchymal stem cells the 'paramedics' of the body because they produce healing factors. They are ideal for repairing damaged tissues in something like spina bifida. Plus, our Good Manufacturing Practice (GMP) facility ensures that the stem cells will be free of infection and properly specialized according to FDA guidelines."
Babies who have this surgery will be closely monitored to evaluate the effects of the stem cells.
Wang and Farmer said the incidence of spina bifida in California, with a disproportionately high rate among babies of Hispanic or Latino descent, makes their study all the more timely and important.
"A successful treatment for MMC would relieve the tremendous emotional and economic cost burden on families," Farmer added. "We know it initially costs approximately $532,000 per child with spina bifida. But the costs are likely several million dollars more due to ongoing treatments, not to mention all the pain and suffering, specialized childcare, and lost time for unpaid caregivers such as parents."
Farmer has been working toward this human clinical trial for spina bifida for more than a decade. She launched the Fetal Care and Treatment Center at UC Davis Children's Hospital with Hirose in preparation for advancing the standard of care. Generous research grants from CIRM also enabled her research team to explore a full range of clinical approaches prior to securing FDA approval for their first human patients.
Patients in the clinical trial will be monitored by the research team for 30 months after they are born to fully assess the stem cell surgical procedure's safety and effectiveness.






