Würzburg chemists have for the first time created a defect in graphene that allows ions to pass through. As they report in 'Nature', this could lead to new applications in water filtration or sensor technology.
Graphene is an extremely thin, flexible and resistant material made of pure carbon. It forms layers that consist of virtually a single layer of carbon atoms. To make graphene as thick as a human hair, thousands of such layers would have to be stacked on top of each other.
Many researchers are working intensively on graphene. There is a good reason for this, as the special properties of the material promise new applications, for example in electronics or energy technology.
Making Graphene Permeable to Other Molecules
It is particularly interesting for scientists to be able to control the permeability of graphene for different substances: 'So-called defects can be created in the carbon lattice of graphene. These can be thought of as small holes that make the lattice permeable to gases,' says chemistry professor Frank Würthner from Julius-Maximilians-Universität (JMU) Würzburg in Bavaria, Germany.
Permeability to other substances, such as ions like fluoride, chloride or bromide, has not yet been observed. 'However, this would be of fundamental scientific interest for applications such as the desalination of water, the detection or purification of mixtures of substances,' explains the Würzburg professor.
Defect Allows Ions to Pass Through: Publication in Nature
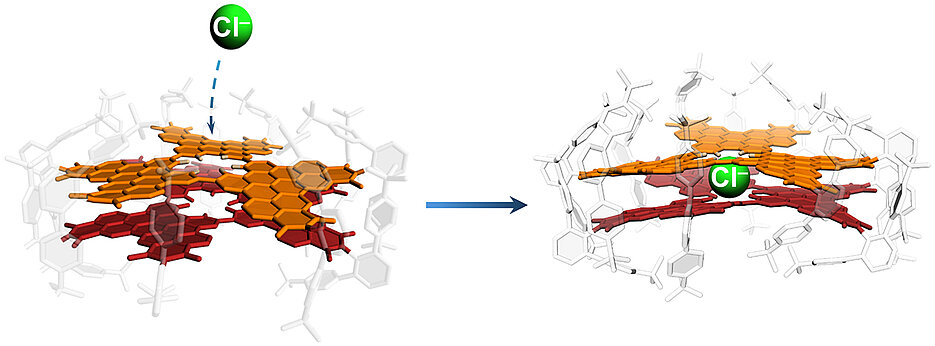
For the first time, a team led by Frank Würthner has now created a model system with a defect that allows the halides fluoride, chloride and bromide to pass through, but not iodide. This was achieved in a stable double layer consisting of two nanographenes that encloses a cavity. The penetrated halide ions are bound in this cavity so that the time required for entry could be measured. The results have been published in the journal Nature.
Chloride is a component of common salt, is found in seawater and plays an important role in life processes in all organisms. 'The proof of a high permeability for chloride by single-layer nanographene and a selective binding of halides in a double-layer nanographene brings some applications closer,' says Dr Kazutaka Shoyama, who initiated and led the project together with Frank Würthner. Such applications include water filtration membranes, artificial receptors and chloride channels.
Larger Stacks of Nanographenes are the Next Goal
In the next step, the Würzburg chemists want to build larger stacks of their nanographenes. They want to use them to investigate the flow of ions - and thus a process that also takes place in a similar form in biological ion channels.
This research was carried out at the Institute of Organic Chemistry and the Center for Nanosystems Chemistry at JMU. The work was funded by the German Research Foundation (DFG) as part of two grants for the development of nanographenes equipped with imide groups.
Publication
Bilayer nanographene reveals halide permeation through a benzene hole. M. A. Niyas, Kazutaka Shoyama, Matthias Grüne & Frank Würthner, Nature, 15 January 2025, DOI: 10.1038/s41586-024-08299-8, https://www.nature.com/articles/s41586-024-08299-8






