Groundwater accounts for around 30 per cent of the world's freshwater, making it an important resource for addressing current global issues, such as world population growth, agricultural intensification and increased water use in different sectors like oil and gas extraction and mining, apparel and textile manufacturing and livestock farming. To protect groundwater from the threats of overextraction and pollution, and to manage it sustainably for the future, it is essential to understand where groundwater in specific locations is originating from, what is its quality and how quickly it replenishes. Scientists can perform this kind of research by analyzing the water 'fingerprints' called "isotopes", which are variations of atoms in the water molecule.
What is groundwater?
Groundwater is water found underground. It can be hidden in the cracks and spaces within rocks and sediments, forming an underground resource, hosted in what is known as an "aquifer". Depending on the characteristics or the aquifer, groundwater can be extracted, using pumping wells, for irrigation, drinking and industrial water supply and other human activities.
How are aquifers formed and why should we use them wisely?
Groundwater is part of the water cycle. Following rainfall, some water soaks into the soil and, driven by gravity, migrates downwards continuously through the subsoil and moves until it is eventually stopped by compact, impermeable rock, called an aquiclude. Many aquifers are connected to, and fed by, rivers and other surface water bodies, during the dry season. In the wet season, this system can be reversed with groundwater moving back into rivers and lakes and replenishing them.
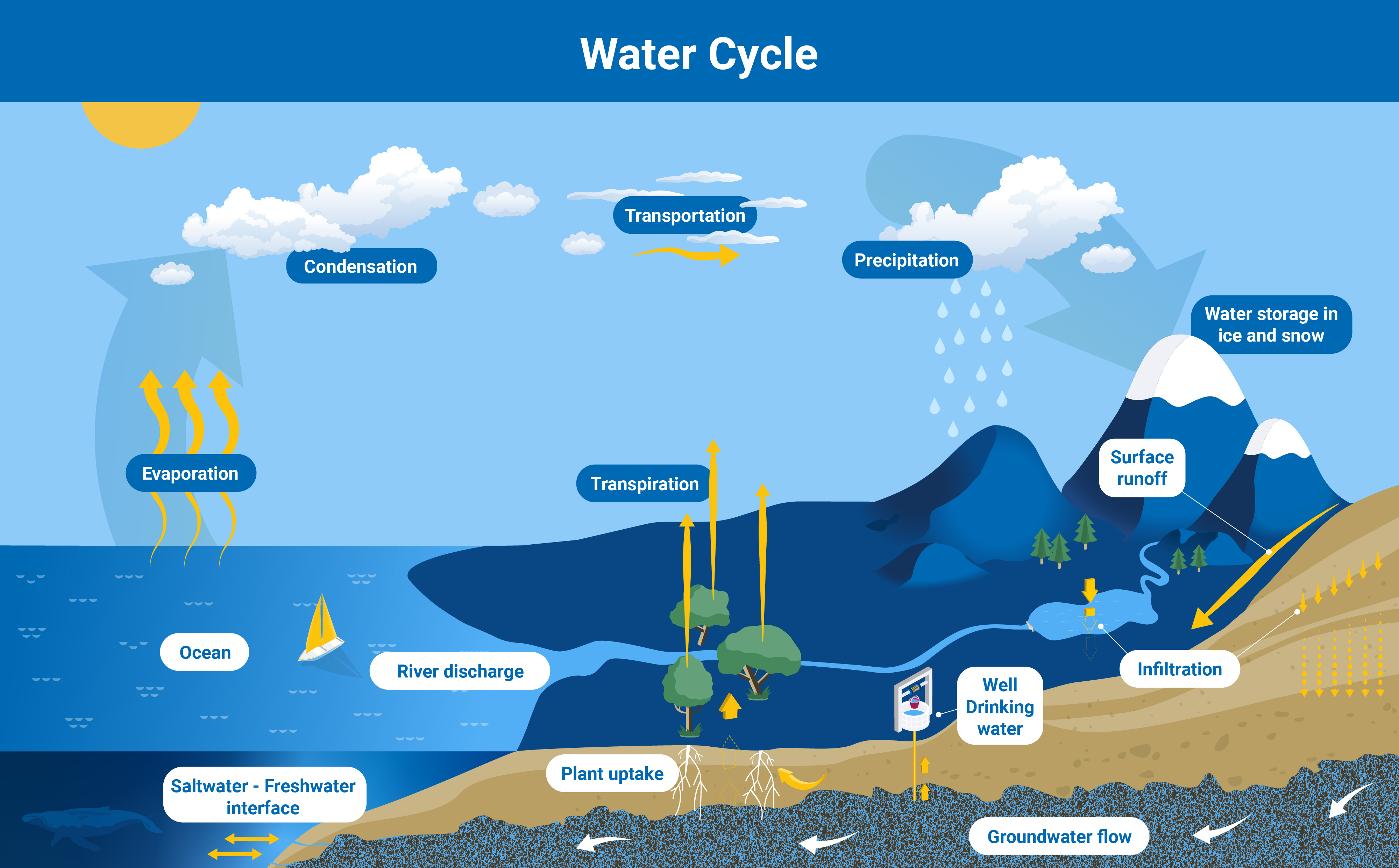
Aquifers are an integral part of the water cycle, and their replenishment rate depends on rainfall, among other factors (Infographic: Adriana Vargas/IAEA).
The rate at which an aquifer is replenished depends on the climate and environment in the location where recharge is happening. Aquifers in an area of low rainfall might take centuries to get refilled. In contrast, shallow aquifers in an area of substantial rainfall may be replenished almost immediately. Thus, climate change, which results in more intense droughts, but also more intense localised rainfall, has an impact on how fast aquifers refill and, by extension, on how much groundwater people can use sustainably.
The intensive use of groundwater for human activities, such as agriculture and industry, at a scale that exceeds the speed at which aquifers refill, may put at risk not only the integrity of the aquifers, which risk collapse if they are drained, but also the global amount of water that people can use, because groundwater constitutes an important part of the world's available freshwater.
Additionally, groundwater may not always be clean enough for human use. Human activities carried out on the surface, such as sewage disposal and the overuse of pesticides and fertilizers, including animal manure, are among the main sources of contamination and pollution of groundwater. Knowing the origin of pollutants, therefore, is the first step toward addressing problems of water quality.
What are isotopes and how can they help scientists understand water?
The water molecule is composed of atoms of oxygen and hydrogen. Some variations of the atoms of the same chemical element, called isotopes, can be used to study the water cycle, including groundwater.
Isotopes are atoms of the same element with the same number of protons but a different number of neutrons.
Different "isotopic" techniques are used to measure isotope amounts and proportions, and to trace their origin, history, sources and interactions in the environment.
Water has a different or unique isotopic "fingerprint", or "isotopic signature", depending on where it comes from. Scientists analyze isotopes to track the movement and pollution sources of water along its path through the water cycle.
How do scientists use isotopes to establish whether groundwater is being overused?
Scientists use isotopes in large-scale studies on water, to assess its amount, age, and origins, and to establish whether the amount being used by people is sustainable.
For example, radioisotopes naturally present in groundwater, such as tritium, carbon-14, and noble gases helium-3, helium-4 and krypton-81, are used to learn more about how old groundwater is and the timescales of groundwater flow. By analyzing the concentration of different combinations of both stable and radio-isotopes, scientists can calculate when exactly the water is recharged in aquifers, how fast groundwater flows, and how long it takes to replenish. With this data, it is possible to establish, for example, whether or not agricultural activities in a specific area are demanding an amount of groundwater that will not be replenished fast enough to sustain irrigation needs in the long run.
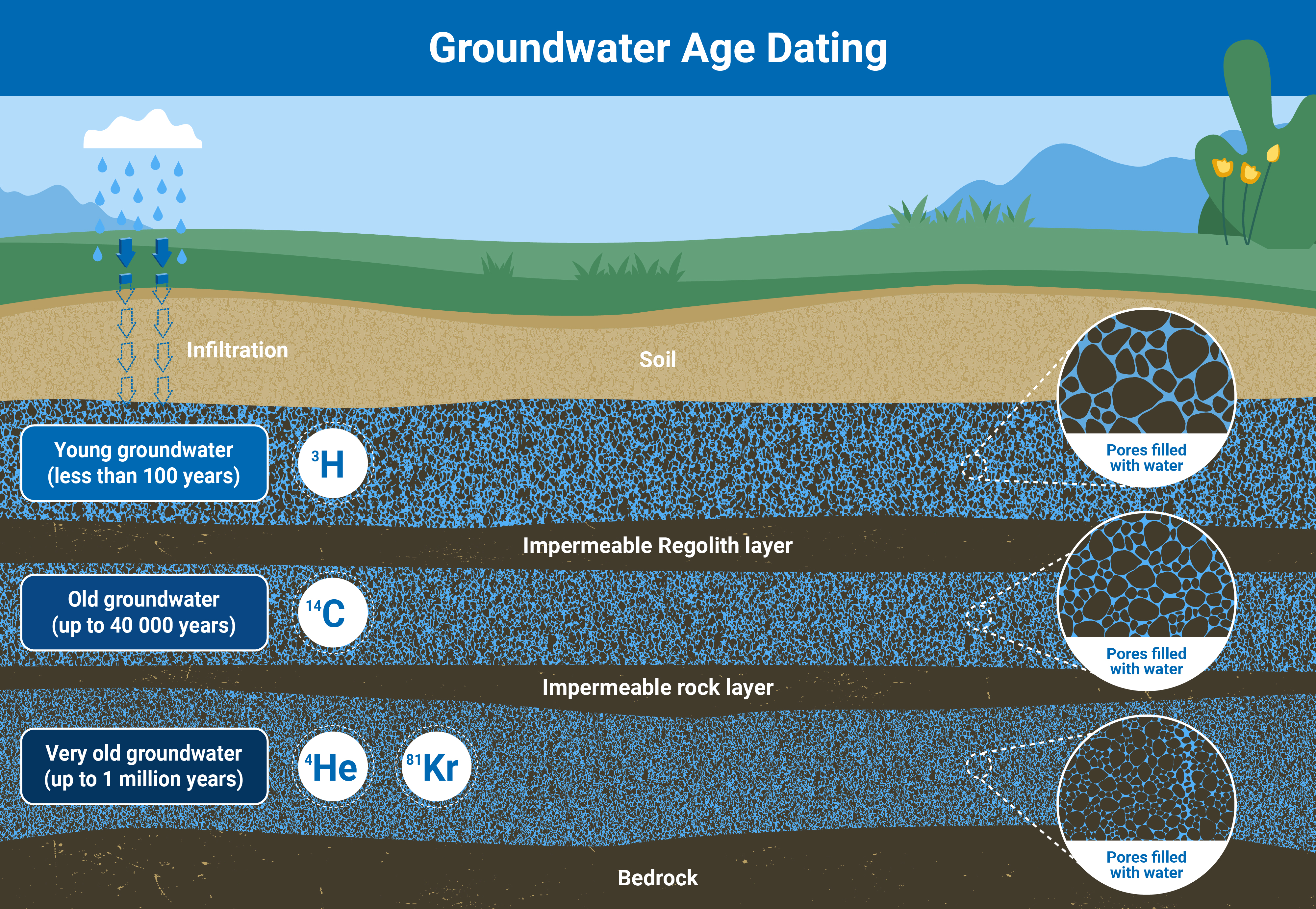
By analyzing the isotopes in groundwater, scientists can establish how old the water is, and deduce how long it will take for an aquifer to recharge based on how much water is being pumped for human activities (Infographic: Adriana Vargas/IAEA).
How do scientists use isotopes to study groundwater pollution?
Scientists use specific isotopes like nitrogen-15, oxygen-18, and sulfur-34 to identify pollutants such as nitrate and sulphates. They also use these isotopes to establish whether the groundwater in a specific location is safe for human use.
For example, scientists can establish whether water contaminated with an excessive amount of nitrate is being polluted by either human waste or by fertilizers. Nitrate ions are made up of nitrogen and oxygen, and nitrogen has two isotopes while oxygen has three. The ratio of these isotopes is different in human waste and in fertilizers. Therefore, the source of pollution can be identified based on these isotopic differences. Knowing the origins of pollutants is a milestone in addressing problems with water quality and working toward the sustainable management of water resources.
What is the role of the IAEA?
- The IAEA uses isotope hydrology to support Member States in water resources assessment and sustainable water management. The Agency also provides assistance and training to laboratories and scientists on analytical services through its Isotope Hydrology Laboratory.
- Offering a wide range of courses, the IAEA provides training on the fundamentals of isotope hydrology and isotopic analyses of stable isotopes, tritium and noble gases.
- Through its technical cooperation programme, the IAEA collaborates closely with its Member States to improve the availability and sustainability of freshwater resources through science-based, comprehensive water resources assessments.
- Partnering with the World Meteorological Organization, the IAEA operates the Global Network of Isotopes in Precipitation, which contains scientific advice, logistics and technical support in isotope hydrology.







