The recent years have witnessed the remarkable progress of chimeric antigen receptor T (CAR-T) cell therapy in the treatment of hematological malignancies. However, unexpected serious toxicities such as cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome still hinder the therapeutic application. Furthermore, the role of the intestinal microbiome in treating the malignancies has raised great attention and the intestinal microbiome is found to affect the efficacy of immunotherapy, such as PD-1 and CTLA-4. Thus, how to improve the therapeutic efficacy of CAR-T therapy by targeting the gut microbiome raises great interests.
On September 10, 2022, Prof. HUANG He and Prof. LI Mingding from the First Affiliated Hospital, Zhejiang University School of Medicine co-published an article entitled CAR-T cell therapy-related cytokine release syndrome and therapeutic response is modulated by the gut microbiome in hematologic malignancies in the journal Nature Communications. This study unravels the link between the gut microbiome and CAR-T therapy-related responses, which may aid inimproving the therapeutic efficacy of CAR-T therapy.
The researchers studied the relationship between the gut microbiome and CAR-T cell therapy-related CRS and therapeutic responses in relapsed and refractory multiple myeloma patients. By applying 16S rRNA sequencing technology and metabolic liquid chromatography mass spectrometry, the researchers discovered that patients descended dramatically in alpha diversity after CAR-T infusion, especially the patients with partly remission. In addition, bacteria, such as Bifidobacterium, Faecalibacterium, Sutterella, and Ruminococcus, were enriched in complete remission patients after CAR-T infusion, indicative of the correlation between bacterial abundance composition and therapeutic response. Moreover, associations between CRS grade and microbial composition were identified, with patients with severe CRS exhibiting higher abundance of Bifidobacterium, Leuconostoc, and Lactococcus.
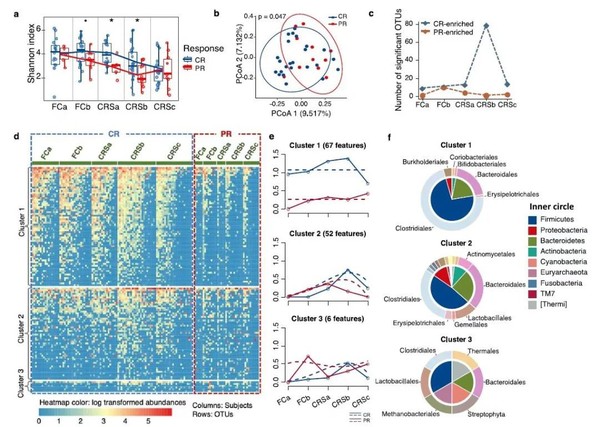
Figure 1 Association of compositional differences in gut microbiome with responses to CAR-T therapy in multiple myeloma patients
Furthermore, through time-course differential analysis, the study revealed correlation of gut microbial functions with CAR-T therapy and identified differential pathways related to RNA polymerase function, amino acid metabolism, fatty acid metabolism, and glutathione metabolism between patients in complete remission and those in partial remission. Additionally, the pathways, such as antibiotic synthesis, lipoic acid metabolism, amino acid metabolism, and nucleic sugar metabolism, differed among patients with different grades of CRS. Besides, the study further analyzed the samples of the validation group by applying metabolic liquid chromatography mass spectrometry. The results showed that the metabolites, such as desoxycortone, L-phenylalanine, L-cystine and choline, were dramatically enriched in patients with complete remission, while phosphocreatine which annotated to arginine and proline metabolism were found to be significantly enriched in patients with severe CRS.
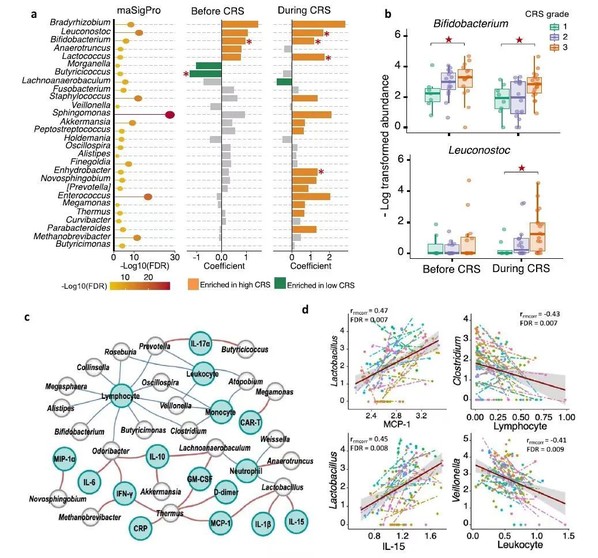
Figure 2 Compositional differences between subjects with different CRS grades
in multiple myeloma patients.
It is the first study around the world to reveal the relationship and mechanisms between the gut microbiome and CAR-T cell therapy-associated effects in patients with relapsed or refractory multiple myeloma. It comprehensively describes how the gut microbiome influences the efficacy of the immunotherapy and CAR-T-related CRS. The study may guide therapeutic intervention to increase efficacy and decrease the toxicity in patients receiving CAR-T therapy.






