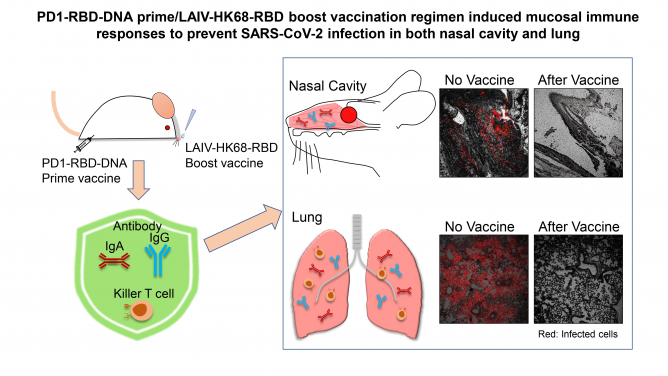
Researchers at the Department of Microbiology and State Key Laboratory of Emerging Infectious Diseases, LKS Faculty of Medicine of The University of Hong Kong (HKUMed) have conducted a comprehensive study for identifying an effective vaccine regimen in preventing SARS-CoV-2 nasal infection. The study demonstrated that a combination of intramuscular PD1-based receptor-binding domain (RBD) DNA vaccine (PD1-RBD-DNA) prime and intranasal live attenuated influenza-based vaccine (LAIV-HK68-RBD) boost vaccination regimen induced the strongest mucosal broadly neutralising antibodies and lung resident memory CD8 T cells, which prevented live SARS-CoV-2 nasal challenges in two animal models. The full research article is now online in the journal of EBioMedicine published by The Lancet [link to the publication].
Background
The COVID-19 pandemic has resulted in over 275 million of infections with nearly 5.36 million deaths by now, yet few vaccines approved for emergency use can induce sufficient mucosal protection for preventing robust SARS-CoV-2 nasal infection. Although the current vaccination reduced rates of hospitalisation, severity and death significantly, these vaccines are much less effective in preventing SARS-CoV-2 respiratory transmission, which has posted great challenges for the pandemic control. With continuous emergence of SARS-CoV-2 variants of concerns including the rapidly spreading of immune escape Omicron strain, it is urgent to discover a more effective vaccine strategy to block or reduce nasal transmission of SARS-CoV-2.
Research methods and findings
In this HKUMed study, substantially higher systemic and mucosal antibodies IgA/IgG and lung resident polyfunctional memory CD8 T cells were induced mainly by the heterologous combination regimen as compared with current COVID-19 vaccination regimens. When two vaccinated mouse models were challenged at the memory phase, 35 days after the second vaccination, prevention of robust SARS-CoV-2 infection in nasal turbinate was achieved primarily by the heterologous combination regimen besides consistent protection in lungs. The new regimen-induced antibodies also cross-neutralised many pandemic variants of concerns tested including Alpha, Beta and Delta. The findings provided the proof-of-concept that vaccine-induced robust mucosal immunity is necessary for preventing SARS-CoV-2 nasal infection, which has significant implication for ending the ongoing COVID-19 pandemic.
Significance of the study
'The findings suggested that the clinical development of our two HKU vaccines remains a top priority for eliminating the uncontrolled spread of COVID-19 pandemic. We are currently testing the influenza-based nasal spray vaccine and the DNA vaccine in humans,' remarked Professor Yuen Kwok-yung, Henry Fok Professor in Infectious Diseases and Chair of Infectious Diseases, Department of Microbiology, HKUMed, who is currently leading the clinical trials of these two vaccines in Hong Kong.
'The biggest challenge for our COVID-19 vaccine development is that we do not have a vaccine manufacturing plant in Hong Kong, which has delayed the translation of scientific discovery into clinical use. Now, we face the same challenge after we have already made the Omicron-targeted DNA vaccine for timely clinical development,' said Professor Chen Zhiwei, Director of the AIDS Institute, Professor of Department of Microbiology, HKUMed, who co-led the research.
'We believe that using nasal spray vaccination to build up protection in the upper respiratory tract is the key strategy to reduce transmission of SARS-CoV-2 and important for the ultimate control of COVID-19 pandemic,' said Professor Chen Honglin, Professor of Department of Microbiology, HKUMed, who co-led the research.
About the research team
The research is co-led by Professor Chen Zhiwei, Director of AIDS Institute, and Professor Chen Honglin, Department of Microbiology, HKUMed; and was conducted primarily by Dr Zhou Runhong, Research Officer; Dr Wang Pui, Scientific Officer; Dr Wong Yik-chun, former Postdoctoral Fellow; Mr Xu Haoran, PhD candidate, at the Department of Microbiology, HKUMed, who shared the first authorship. This team also includes Ms Lau Siu-ying, Technical officer; Dr Liu Li, Research Assistant Professor; Dr Anna Zhang Jinxia, Dr Bobo Mok Wing-yee, Scientific Officers; Dr Rachel Tam Chun-yee, Dr Zhou Dongyan, Postdoctoral Fellows; Ms Peng Qiaoli and Ms Liu Na, Mr Deng Shaofeng, Mr Zhou Biao, PhD candidates; Mr Chan Chun-yin, Technical Officer; Mr Woo Kin-fai, Mr Huang Haode, Mr Du Zhenglong, Mr Yang Dawei, and Mr Au Ka-kit, Research Assistants; Research Assistant; and Professor Yuen Kwok-yung, Henry Fok Professor in Infectious Diseases and Chair of Infectious Diseases, Department of Microbiology, HKUMed.
Acknowledgements
The preclinical study was supported by the Hong Kong Research Grants Council - Collaborative Research Fund (C7156-20G, C1134-20G and C5110-20G), General Research Fund (17107019) and Health and Medical Research Fund (19181052 and 19181012) from the Food and Health Bureau, The Government of the Hong Kong Special Administrative Region; the Hong Kong Health@InnoHK, Innovation and Technology Commission; and the China National Program on Key Research Project (2020YFC0860600, 2020YFA0707500 and 2020YFA0707504); and donations from the Friends of Hope Education Fund. Professor Chen Zhiwei's team was also partly supported by the Hong Kong Research Grants Council - Theme-Based Research Scheme (T11-706/18-N). The GMP production of the DNA vaccine and the live attenuated influenza-based vaccine were supported by Shenzhen Science and Technology Program (JSGG20200225151410198) with matching fund from the Shenzhen Immuno Cure BioTech Limited and Outbreak Response to Novel coronavirus (COVID-19) by the Coalition for Epidemic Preparedness Innovations (CEPI), respectively.
About the Department of Microbiology, HKUMed
The academic staff of Department of Microbiology are actively involved in clinical service and basic research. Postgraduate students may pursue studies on various aspects of microbiology and infectious diseases leading to an MPhil or PhD degree. The Master of Medical Sciences programme offers an opportunity to postgraduates interested in more in-depth studies on the biomedical aspects of clinical microbiology and infectious disease. In addition, the clinical staff of the Department also participate in the training of clinical microbiologists in Hong Kong and Shenzhen. The Infectious Disease Courses and Postgraduate Diploma Programme provides a unique avenue for the training of qualified medical practitioners in infectious diseases.
To promote knowledge exchange, research activities at the Department of Microbiology, HKUMed can be viewed through http://www.microbiology.hku.hk/.