An organic catalyst offers chemists precise control over a vital step in activating hydrocarbons.
An artist's rendition of the new catalytic method for asymmetric fragmentation of cyclopropanes. (Credit: YAP Co., Ltd.)
Researchers at Hokkaido University in Japan have made a significant breakthrough in organic chemistry by developing a novel method to activate alkanes, which are compounds that play a crucial role in the chemical industry. The new technique, published in Science, makes it easier to convert these building blocks into valuable compounds, offering advances in the production of medicines and cutting-edge materials.
Alkanes are a primary component of fossil fuels and are also vital building blocks in the production of various chemicals and materials, such as plastics, solvents, and lubricants. But their strong carbon-carbon bonds make them quite stable and inert, presenting a challenge for chemists trying to convert them into useful compounds. To address this issue, scientists have focused on cyclopropanes, a specific type of alkane with a ring structure that makes them more reactive than other alkanes.
Many of the existing techniques for breaking down long-chain alkanes, known as cracking, tend to generate a mixture of molecules, making it challenging to isolate the desired products. This challenge arises from the cationic intermediate, a carbonium ion, which has a carbon atom bonded to five groups instead of the three typically described for a carbocation in chemistry textbooks. This makes it extremely reactive and difficult to control its selectivity.
The research team discovered that a particular class of confined chiral Brønsted acids, called imidodiphosphorimidate (IDPi), could address this problem. IDPi's are very strong acids that can donate protons to activate cyclopropanes and facilitate their selective fragmentation within their microenvironments. The ability to donate protons within such a confined active site allows for greater control over the reaction mechanism, improving efficiency and selectivity in producing valuable products.
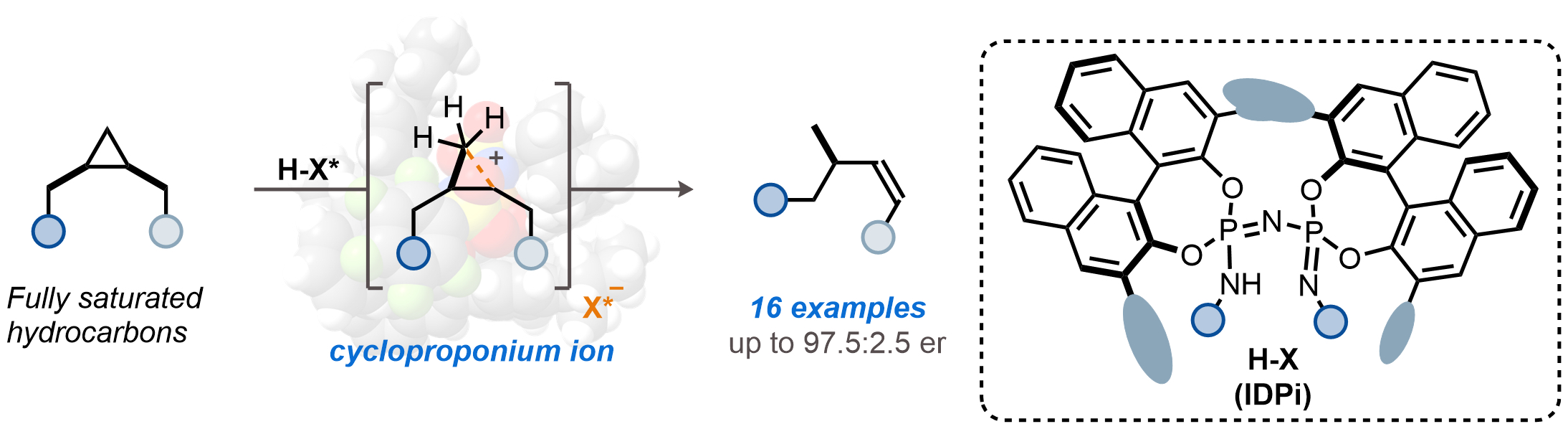
Confined chiral Brønsted acids, IDPi, are used to efficiently convert cyclopropanes into valuable compounds by donating protons during the reaction. (Ravindra Krushnaji Raut, et al. Science. October 10, 2024)
"By utilizing a specific class of these acids, we established a controlled environment that allows cyclopropanes to break apart into alkenes while ensuring precise arrangements of atoms in the resulting molecules," says Professor Benjamin List, who led the study together with Associate Professor Nobuya Tsuji of the Institute for Chemical Reaction Design and Discovery at Hokkaido University, and is affiliated with both the Max-Planck-Institut für Kohlenforschung and Hokkaido University. "This precision, known as stereoselectivity, is crucial for example in scents and pharmaceuticals, where the specific form of a molecule can significantly influence its function."
The success of this method stems from the catalyst's ability to stabilize unique transient structures formed during the reaction, guiding the process toward the desired products while minimizing unwanted byproducts. To optimize their approach, the researchers systematically refined the structure of their catalyst, which improved the results.
"The modifications we made to certain parts of the catalyst enabled us to produce higher amounts of the desired products and specific forms of the molecule," explains Associate Professor Nobuya Tsuji, the other corresponding author of this study. "By using advanced computational simulations, we were able to visualize how the acid interacts with the cyclopropane, effectively steering the reaction toward the desired outcome."
The researchers also tested their method on a variety of compounds, demonstrating its effectiveness in converting not only a specific type of cyclopropanes but also more complex molecules into valuable products.
This innovative approach enhances the efficiency of chemical reactions as well as opens new avenues for creating valuable chemicals from common hydrocarbon sources. The ability to precisely control the arrangement of atoms in the final products could lead to the development of targeted chemicals for diverse applications, ranging from pharmaceuticals to advanced materials.

Clockwise from bottom left: Nobuya Tsuji, Ravindra Krushnaji Raut, Satoshi Maeda, Shuta Kataoka, Satoshi Matsutani and Benjamin List of the research team. (Photo: Benjamin List)
Original Article:
Ravindra Krushnaji Raut, et al. Catalytic asymmetric fragmentation of cyclopropanes. Science. October 10, 2024.
DOI: 10.1126/science.adp9061
Funding:
This research was supported by the Institute for Chemical Reaction Design and Discovery (ICReDD), which was established by the World Premier International Research Initiative (WPI), MEXT, Japan; the List Sustainable Digital Transformation Catalyst Collaboration Research Platform offered by Hokkaido University; the Japan Society for the Promotion of Science (JSPS), JSPS KAKENHI (21H01925, 22K14672); the Japan Science and Technology Agency (JST) SPRING (JPMJSP2119); the Max Planck Society; the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany's Excellence Strategy (EXC 2033-390677874-RESOLV); the European Research Council (ERC) [European Union's Horizon 2020 research and innovation program "C−H Acids for Organic Synthesis, CHAOS," Advanced Grant Agreement no. 694228; and European Union's Horizon 2022 research and innovation program "Early Stage Organocatalysis, ESO," Advanced Grant Agreement no. 101055472]; and the Fonds der Chemischen Industrie.






