An organism as a tenant in another - in biology, this often works quite well. ETH researchers have now shed light on how such a partnership of a cell in a cell can establish.
In brief
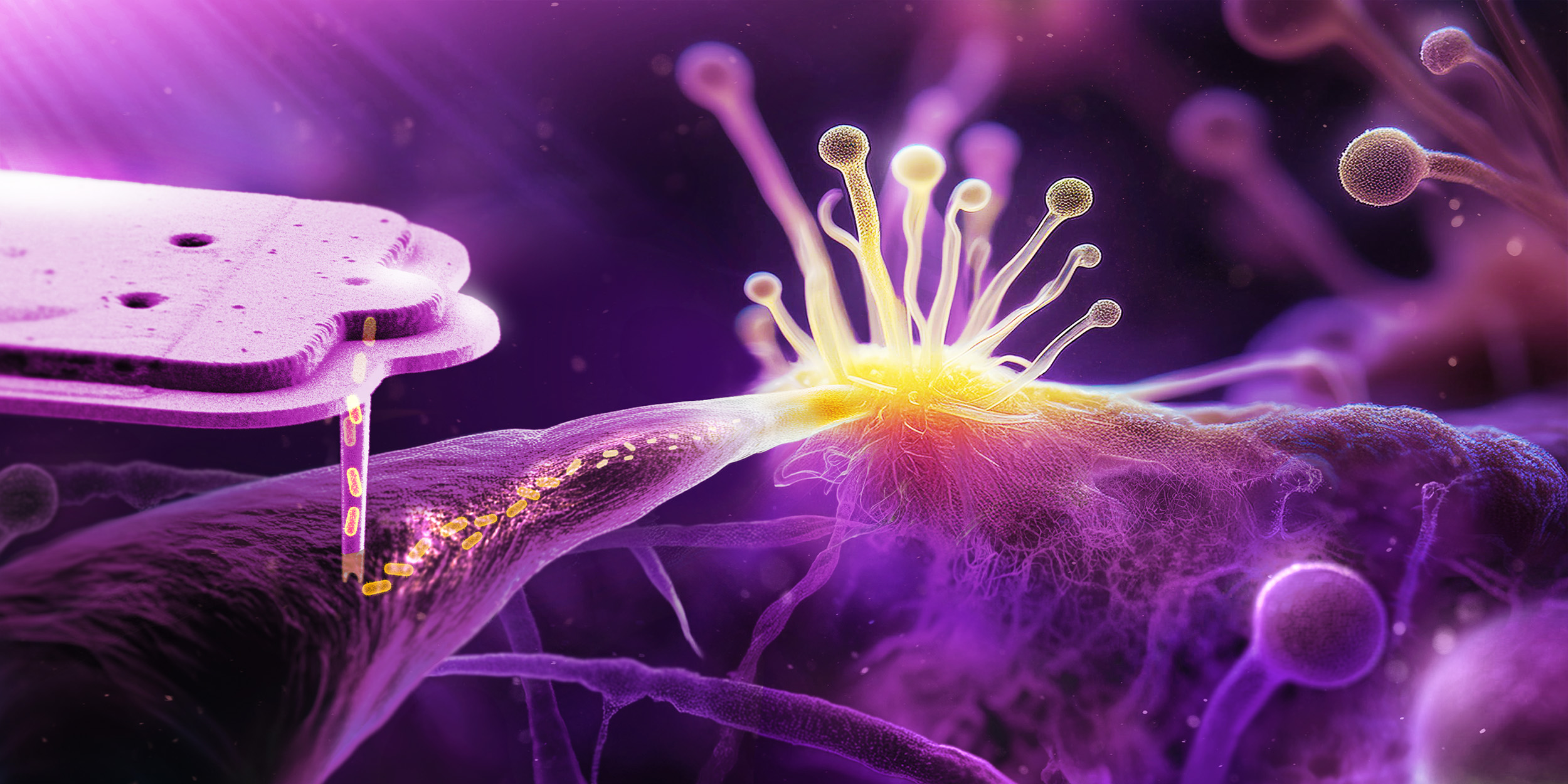
- ETH researchers investigated the beginning of endosymbiosis, the stable coexistence of a bacterium in a fungus. To do this, they injected bacteria into cells of a fungus.
- They observed that the implanted bacteria were able to both multiply within the host cells and be passed on to the next generation.
- The cohabiting partners adapted to each other and eventually both benefited.
Endosymbiosis is a fascinating biological phenomenon in which an organism lives inside another. Such an unusual relationship is often beneficial for both parties. Even in our bodies, we find remnants of such cohabitation: mitochondria, the powerhouses of our cells, evolved from an ancient endosymbiosis. Long ago, bacteria entered other cells and stayed. This coexistence laid the foundation for mitochondria and thus the cells of plants, animals, and fungi.
What is still poorly understood, however, is how an endosymbiosis as a lifestyle actually arises. A bacterium that more or less accidentally ends up in a completely different host cell generally has a hard time. It needs to survive, multiply, and be passed on to the next generation. Otherwise, it dies out. And to not harm the host, it must not claim too many nutrients for itself and grow too quickly. In other words, if the host and its resident cannot get along, the relationship ends.
To study the beginnings of such a special relationship between two organisms, a team of researchers led by Julia Vorholt, Professor of Microbiology at ETH Zurich, initiated such partnerships in the laboratory. The scientists observed what exactly happens at the beginning of a possible endosymbiosis. They have just published their study in the scientific journal external page Nature.
"The fact that the bacteria are actually transmitted to the next generation of fungi via the spores was a breakthrough in our research."Gabriel Giger
Enforcing cohabitation
For this work, Gabriel Giger, a doctoral student in Vorholt's laboratory, first developed a method to inject bacteria into cells of the fungus Rhizopus microsporus without destroying them. He used E. coli bacteria on the one hand and bacteria of the genus Mycetohabitans on the other. The latter are natural endosymbionts of another Rhizopus fungus. For the experiment, however, the researchers used a strain that does not form an endosymbiosis in nature. Giger then observed what happened to the enforced cohabitation under the microscope.
After the injection of the E. coli bacteria, both the fungus and the bacteria continued to grow, the latter eventually so rapidly that the fungus mounted an immune response against the bacteria. The fungus protected itself from the bacteria by encapsulating them. This prevented the bacteria from being passed on to the next generation of fungi.
Bacteria enter the spores
This was not the case with the injected Mycetohabitans bacteria: While the fungus was forming spores, some of the bacteria managed to get into them and thus were passed on to the next generation. "The fact that the bacteria are actually transmitted to the next generation of fungi via the spores was a breakthrough in our research," says Giger.
When the doctoral student allowed the spores with the resident bacteria to germinate, he found that they germinated less frequently and that the young fungi grew more slowly than without them. "The endosymbiosis initially lowered the general fitness of the affected fungi," he explains. Giger continued the experiment over several generations of fungi, deliberately selecting those fungi whose spores contained bacteria. This enabled the fungus to recover and produce more inhabited but viable spores. As the researchers were able to show with genetic analyses, the fungus changed during this experiment and adapted to its resident.
The researchers also found that the resident, together with its host, produced biologically active molecules that could help the host obtain nutrients and defend itself against predators such as nematodes or amoebae. "The initial disadvantage can thus become an advantage," emphasizes Vorholt.
Fragile systems
In their study, the researchers show how fragile early endosymbiotic systems are. "The fact that the host's fitness initially declines could mean the early demise of such a system under natural conditions," says Giger. "For new endosymbioses to arise and stabilize, there needs to be an advantage to living together," says Vorholt. The prerequisite for this is that the prospective resident brings with it properties that favor endosymbiosis. For the host, it is an opportunity to acquire new characteristics in one swoop by incorporating another organism, even if it requires adaptations. "In evolution, endosymbioses have shown how successful they ultimately can become," emphasizes the ETH professor.
Reference
Giger GH, Ernst C, Richter I, et al. Inducing novel endosymbioses by implanting bacteria in fungi. Nature, 02 October 2024, doi: external page 10.1038/s41586-024-08010-x






