In an important study for understanding how memories are made, EPFL scientists show that the flexibility of chromatin - packaged DNA inside the cell - plays a crucial role in "deciding" which neurons are involved in forming a specific memory.
When we form a new memory, the brain undergoes physical and functional changes known collectively as a "memory trace". A memory trace represents the specific patterns of activity and structural modifications of neurons that occur when a memory is formed and later recalled.
But how does the brain "decide" which neurons will be involved in a memory trace? Studies have suggested that the inherent excitability of neurons plays a role, but the currently accepted view of learning has neglected to look inside the command center of the neuron itself, its nucleus. In the nucleus, there seems to be another dimension altogether that has gone unexplored: epigenetics.
Inside every cell of a given living organism, the genetic material encoded by the DNA is the same, yet the various cells types that make up the body, like skin cells, kidney cells, or nerve cells each express a different set of genes. Epigenetics is the mechanism of how cells control such gene activity without changing the DNA sequence.
Now, scientists at EPFL led by neuroscientist Johannes Gräff have explored whether epigenetics might affect the likelihood of neurons to be selected for memory formation. Their research on mice, now published in Science, shows that the epigenetic state of a neuron is key to its role in memory encoding. "We are shedding light on the earliest step of memory formation from a DNA-centric level", says Gräff.
Gräff and his team wondered if epigenetic factors could influence the "mnemonic" function of a neuron. A neuron can be epigenetically open when the DNA inside its nucleus is unraveled or relaxed; and closed when the DNA is compact and tight.
They found that it is the open ones that are more likely to be recruited into the "memory trace", the sparse set of neurons in the brain that shows electrical activity when learning something new. Indeed, the neurons that were in a more open chromatin state were also the ones demonstrating higher electrical activity.
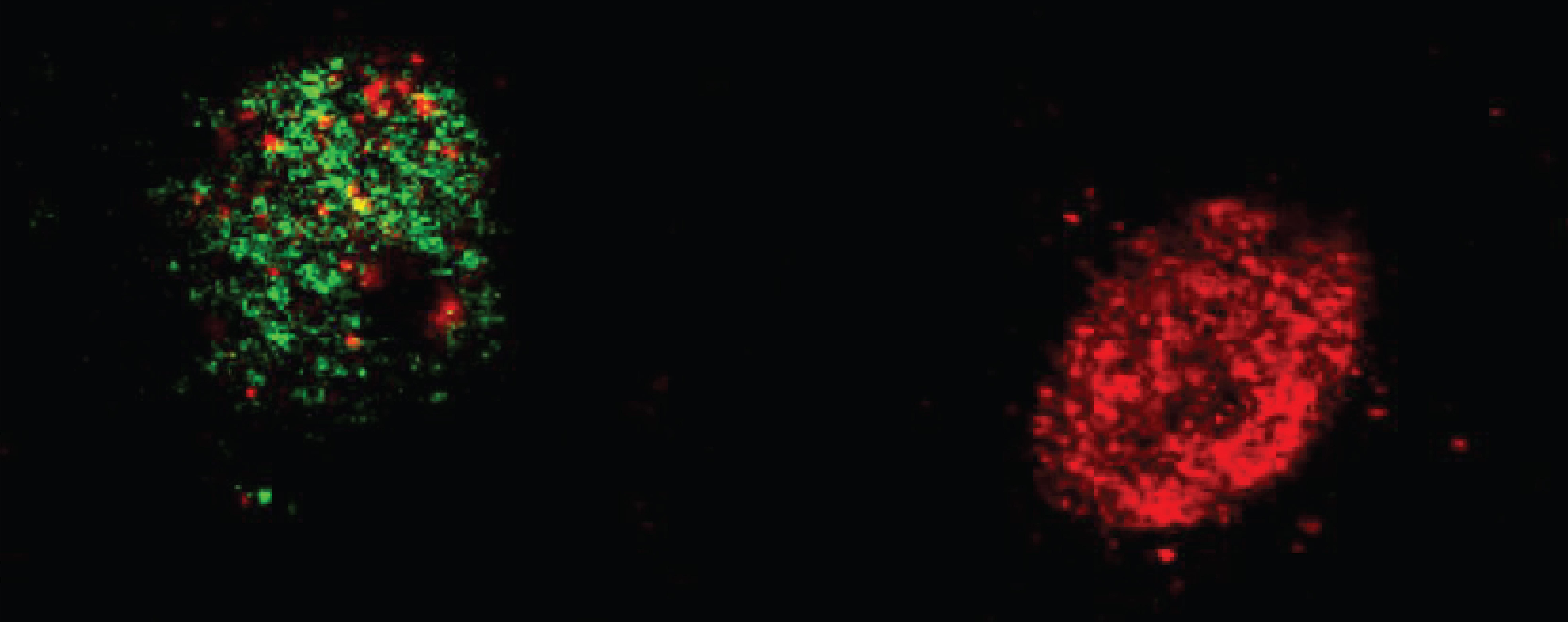
The EPFL scientists then used a virus to deliver epigenetic enzymes to artificially induce openness of the neurons. They found that the corresponding mice learnt much better. When the scientists used the opposite approach to close the neurons' DNA, the mice's ability to learn was cancelled.
The findings open up new ways to understand learning that encompass the neuron's nucleus, and may even lead one day to medication for improving learning. As Gräff explains: "They move away from the dominant neuroscientific view on learning and memory that focuses on the importance of synaptic plasticity, and newly place emphasis on what happens inside the nucleus of a neuron, on its DNA. This is especially important, as many cognitive disorders such as Alzheimer's disease and post-traumatic stress disorder are characterized by epigenetic mechanisms gone wrong."
Other contributors
- EPFL Laboratory of Behavioural Genetics
- EPFL Bioinformatics Competence Center






