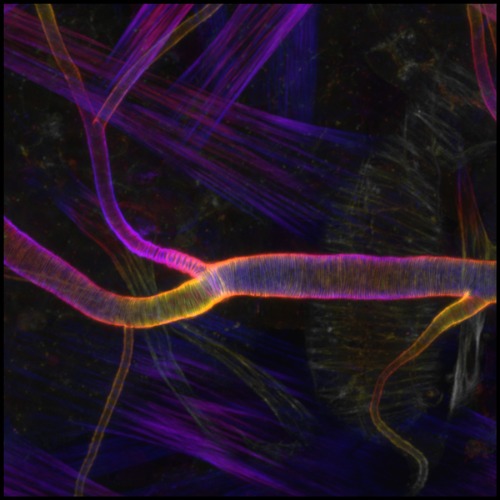
Figure 1: Fluorescent labeling shows the orderly pattern of actin fibers that reinforce channels in the respiratory system of fruit flies. © 2024 RIKEN Center for Biosystems Dynamics Research
RIKEN scientists have developed a model that explains the orderly patterning process of cytoskeletons underlying the formation of a network of tubular structures that supply our bodies with the oxygen and nutrients we need to survive1.
For blood to flow steadily through the circulatory system, our blood vessels need to maintain a uniform internal channel, also known as a lumen. This is achieved through reinforcement with regularly spaced, circumferential cables composed of a protein called actin. Similar actin structures also stabilize the cellular projections that connect neurons within the brain, as well as the tubular network that forms the respiratory system in fruit flies.
However, the processes underlying the formation of these tubular structures remain poorly understood. "It was mystifying how such tubular tissues are formed to have smooth, uniform lumens," says Sayaka Sekine of the RIKEN Center for Biosystems Dynamics Research (BDR).
To explore this question, a team led by Sekine and Mitsusuke Tarama, also of BDR, has used the development of respiratory system, trachea, in flies as a model (Fig. 1).
Preliminary experiments indicated that the lumen-reinforcing actin cables seen in fully formed tubules initially arise from elliptical assemblies of actin, which the researchers termed actin nanoclusters.
To identify the genes that contribute to the formation and organization of these nanoclusters, the team systematically inhibited 119 genes known to encode proteins that interact with actin.
Based on the insights from these and subsequent experiments, the team developed a computer model of the actin assembly process, in which they boiled the system down to a small handful of variables.
"The basic strategy was to make a model as simple as possible, but not too simple," explains Tarama.
Their model revealed that the uneven distribution of stress that arises as tubules expand outward induces a response in a protein called dishevelled-associated activator of morphogenesis (DAAM), which in turn promotes the reorganization of actin nanoclusters into orderly aligned and evenly spaced cables within the tubular lumen.
"The entire transition process from actin nanoclusters to cable formation can be explained by self-organization in expanding tubules," says Sekine.
She and her colleagues now hope to get a better handle on the sensing mechanism that drives this actin fiber assembly. "Clarifying the detailed molecular mechanism may enable more accurate computer modeling and prediction of the higher-order structures of actin in other tubular tissues," says Sekine.
These mechanisms could play a role in the formation of more complex human tissues, including the primitive heart tube, Sekine notes.






