A new proof-of-concept study by researchers at the Herbert Irving Comprehensive Cancer Center (HICCC) shows that changing only a single letter in the DNA code of selected genes in T cells may lead to improved cell therapy. The researchers, led by Benjamin Izar, a member of the HICCC, used novel CRISPR-dependent base editing to "super charge" cell therapies, making them potentially more effective for more patients.
Cell therapies work by re-engineering a patient's own immune cells as a form of immunotherapy. These novel therapies have been successful in particularly difficult to treat cancers, but so far, have yet to have a far-reaching impact for all patients and all cancer types.

Pictured: Benjamin Izar
"While some patients experience durable responses, most do not," says Izar, senior author on the new study, published today in Nature Biotechnology. Each patient's unique tumor microenvironment, the complex and dynamic cells and molecules surrounding the tumor, can suppress immune cell activity and prevent the therapy from being effective. Cancer cell intrinsic factors can also play a role in immunotherapy response and resistance.
"What we do know is that the function of the T cell - the building block for cell therapies - is highly predictive of whether or not the cell product built from it will be effective against treating the patient's cancer," continues Izar.
Previous research has experimented with engineering the genome of T cells with CRISPR-Cas9 technologies to boost T cell activity. Instead of knocking out whole genes, Izar and his colleagues wanted to test whether introducing small changes to the genome - single nucleotide variants using a new technique called base editing, would be sufficient to improve the function of T cells.
"The approach of introducing specific variants could be used with existing cell therapies, where we modify those products to make them more active and less likely to fail in patients," says Izar. "Or we could build entirely new products using these variant proteins as a foundation."
In the study, the researchers performed massively parallel base editing screens across 102 genes to generate thousands of variants. After mapping the impact of these variants, they picked only those that improved multiple hallmarks of T cell activities known to be critical to eliminate cancer.
"We designed this library of over 10,000 different mutations that we can make in all these genes that we know to be important for T cell function," says Zachary Walsh, a graduate student in Izar's lab and the paper's first author. "In our results, we discovered this really interesting series of mutations that actually all cluster together in a couple of regions of the same gene."
Introducing specific mutations of this gene, PIK3CD, into tumor-specific T cells caused them to become "super soldiers" that were able to kill melanoma and leukemia cells more efficiently. They also generated more anti-tumor cytokines that are considered beneficial in the context of an anti-cancer response.
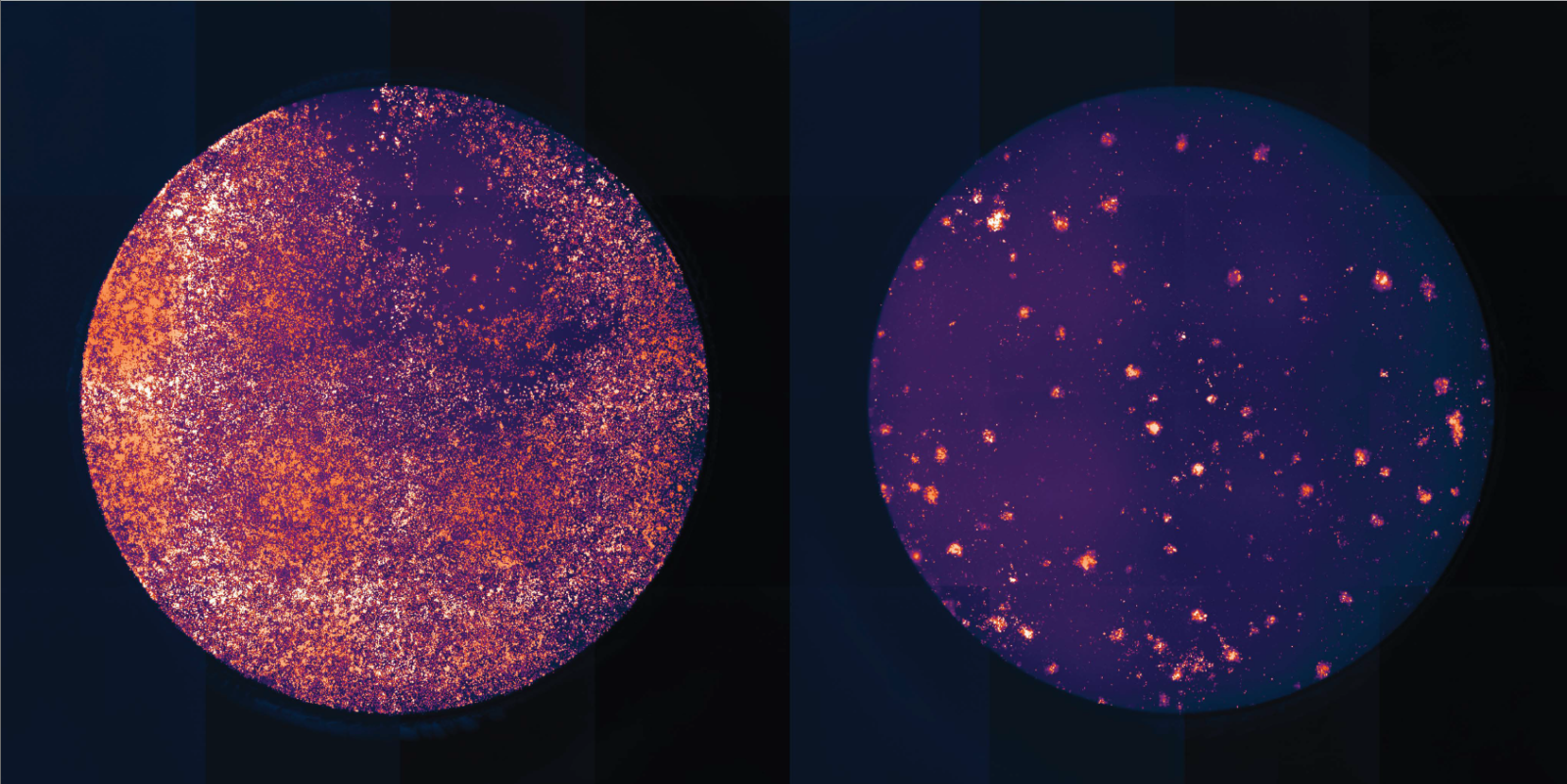
Engineered mutant T cells (right) showing far fewer tumor survivors than un-edited T cells (left).
The work was supported by the Human Tissue Immunity and Immunotherapy Initiative (HTI3), an HICCC initiative that aims to shed light on how the immune system and cancer interact. The goal of HTI3 is to help answer current clinical challenges, like why some patients respond to immunotherapy and others do not, that could lead to new personalized and targeted therapies that harness the full potential of the patient's immune system.
"To generate T-cell super soldiers that can be used in patients, we will likely need to introduce more than one variant in either the same gene or in different genes," Izar says. "There is more work to do, and one of the first things we're testing is whether combinatorial mutations are required for more effective cell therapy."






