Sugar uptake mechanism understanding may have implications for biorefineries.
With the help of genetic manipulation and advanced biophysical tools, an international research team has gained unexpected insight into how a bacterium uptakes sugars derived from plant feedstock.
Their findings were published on Sept. 7 in mBio.
"Efficient sugar uptake is crucial for microbial cell factories, so sugar transporters are important targets for metabolic engineering and synthetic biology development of industrial microorganisms," said co-corresponding author Prof. CUI Qiu from the Qingdao Institute of Bioenergy and Bioprocess Technology (QIBEBT) of the Chinese Academy of Sciences (CAS).
The bacterium, Clostridium thermocellum, has long been a lead contender in the sustainable industrial production of biofuels. Although how the industrial microorganism C. thermocellum uptakes sugars has been of great interest for many years and five potential sugar transporters were discovered in 2009, difficulties with gene manipulation in this bacterium has restricted functional validation.
The Metabolomics Group at QIBEBT led by CUI has developed a variety of tools capable of gene manipulation in C. thermocellum. These tools include cell electroporation instrument, fast gene knockout technique for thermophilic bacteria (Thermotargetron technique), and precise genome editing system, which allow the researchers to analyze which genes produce what physical and functional changes in bacteria, informing how the bacterium breaks down lignocellulose, the main component of plant cell walls, into sugar types that can be used to produce ethanol.
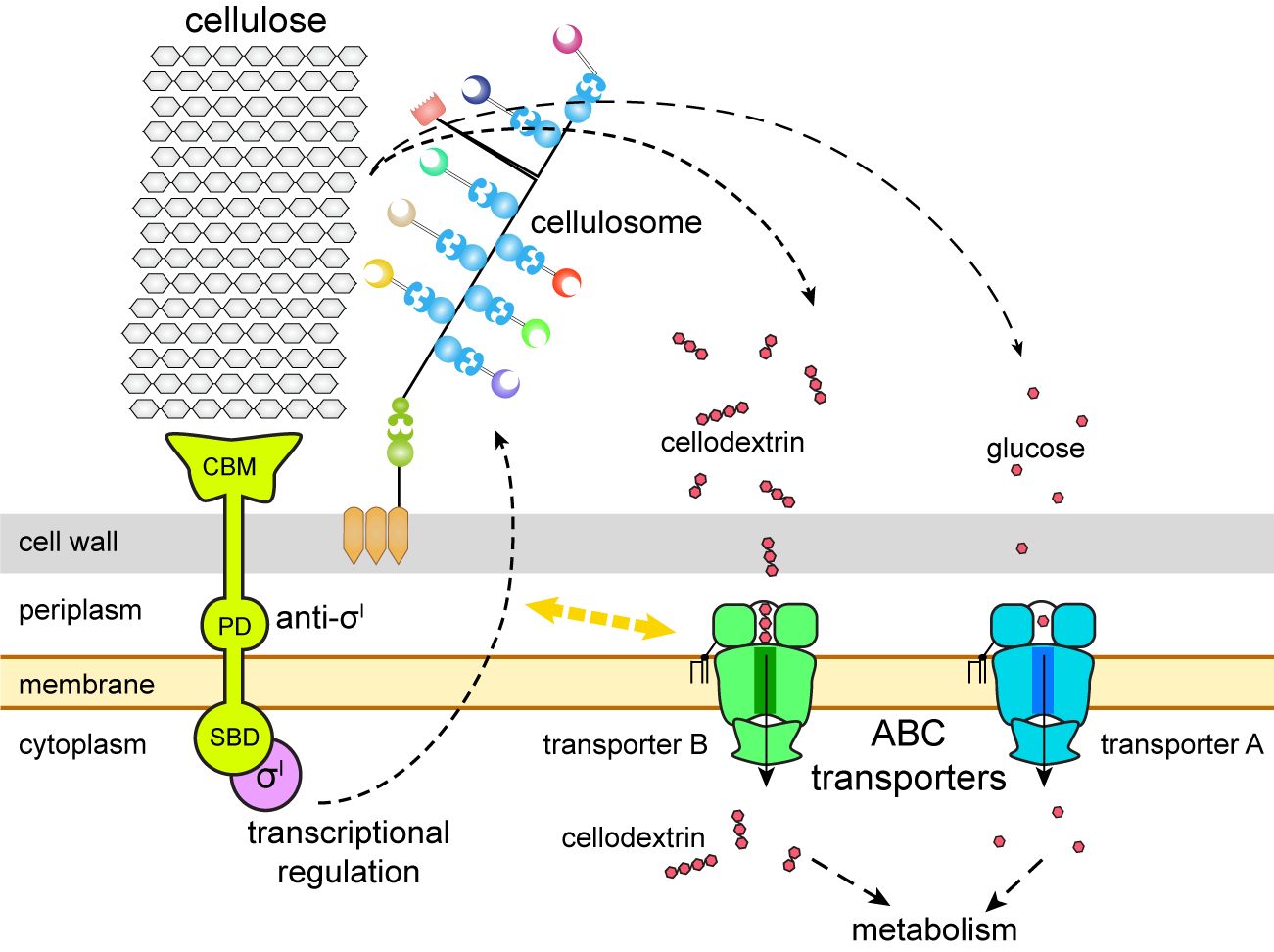
By combining these genetic approaches and various biophysical techniques, the researchers identified the transporters known as B and A as the solo transporters of cellodextrin and glucose, respectively, among the five potential sugar transporters in C. thermocellum. Glucose is a simple sugar molecule, while cellodextrin comprises multiple bonded molecules. "These findings are quite unexpected because many microorganisms are known to have a multiplicity of redundant sugar transporters for their major carbon source," said CUI.
Instead of multiple transporters to collect and move sugars like other microorganisms, C. thermocellum primarily uses transporter B to take up cellodextrins derived from cellulose. This strain also uses transporter A to take up glucose and make use of it, but only after its properly adapted, according to co-corresponding author Prof. FENG Yingang from QIBEBT.
"We also identified an isolated gene, 2554, as the missing subunit in the transporter B gene cluster," FENG said, noting the finding explains how transporter B's mechanism could help utilize lignocellulosic biomass. "In addition, we demonstrated that transporter B couples with the expression regulation of cellulosomes, the protein complexes responsible for producing sugars by degrading plant cell walls."
This finding extends prior research in the field suggesting cellulosome expression is regulated by a group of sigma/anti-sigma factors with substrate-sensing and transcription activity, while is also coupled with sugar transport. And yet the researchers did not fully elucidate the underlying mechanism of this sugar transporter-cellulosome coupling, and the discovery of its existence further bolsters the potential application value of C. thermocellum in lignocellulose bioconversion.
"Although the physiological and evolutionary benefits of the restrictive transporters are still unknown, these findings suggest that engineering sugar transporters in C. thermocellum could be easier than in species with multiple redundant transporters," CUI said.
The researchers said they plan to continue working to understand the key molecular mechanisms of the bacterium, which they will use to inform the development of bioenergy and synthetic biology technologies.