A significant breakthrough in the field of solid-state batteries has been achieved by a research team led by Prof. HU Yongsheng from the Institute of Physics (IOP) of the Chinese Academy of Sciences (CAS). Their study of a novel viscoelastic inorganic glass (VIGLAS) electrolyte has been published in Nature Energy.
Often hailed as the next disruptive battery technology, solid-state batteries offer a promising solution to the safety concerns associated with conventional liquid lithium-ion batteries while significantly increasing energy density. This technological achievement has the potential to revolutionize key industries, including electric vehicles, energy storage, and mobile devices. However, the practical implementation of solid-state batteries is hindered by several limitations related to interface stability and manufacturing costs. For example, organic polymer solid-state batteries exhibit impressive mechanical stability at the interface, but they fall short in terms of chemical stability. This limitation restricts their energy density, primarily due to compatibility issues with high-voltage cathodes.
On the other hand, commercially viable inorganic sulfide solid-state batteries, while promising, has the disadvantage of high manufacturing costs. Moreover, they require operation at extremely high pressures, often reaching several tens of atmospheres, posing significant challenges on the path to commercialization. Therefore, the search for a novel electrolyte material that can effectively address these issues becomes paramount in the pursuit of advancing solid-state battery technology.
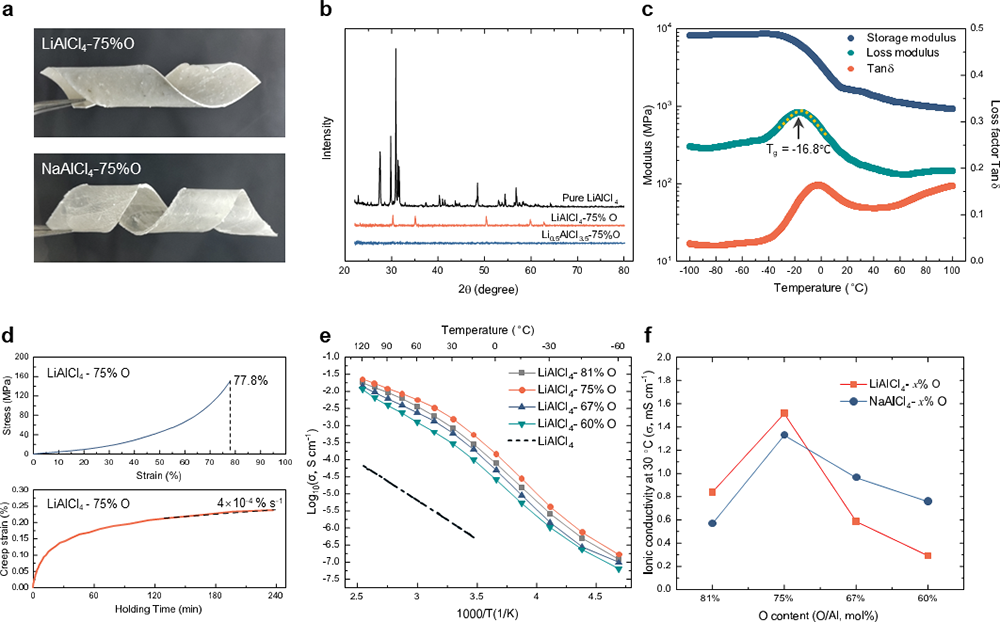
In this study, the researchers have succeeded in transforming of delicate room-temperature molten salts, namely LiAlCl4 and NaAlCl4, into viscoelastic glasses called LiAlCl2.5O0.75 (LACO) and NaAlCl2.5O0.75 (NACO), by introducing oxygen atoms in place of certain chlorine atoms. What sets this material apart is its remarkable ability to bend and fold with ease at room temperature, challenging the previous assumption that inorganic solid electrolytes couldn't exhibit the mechanical flexibility associated with organic electrolytes. This groundbreaking discovery paves the way for an entirely new frontier in solid-state electrolyte development.
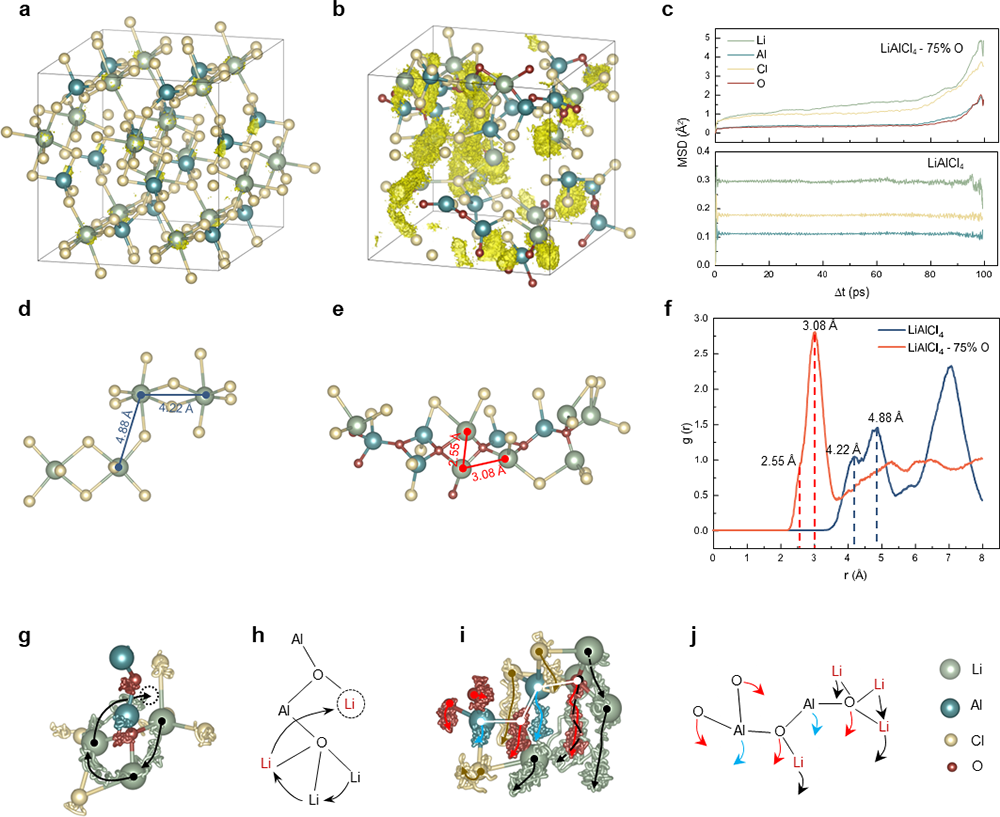
They have revealed both the formation and ion conduction mechanisms of these inorganic glasses, known as VIGLAS materials. These materials exhibit a glass transition temperature (Tg) lower than room temperature, resulting in a viscoelastic behavior reminiscent of polymers at ambient conditions. This low Tg can be attributed to the balanced oxygen-chlorine ratio, where oxygen bridges play a crucial role in forming appropriately sized Al-O-Al networks. These networks, in turn, limit atomic rearrangement during condensation.
In addition, the presence of trace amounts of uncoordinated LixAlCl3+x, acting as "plasticizers," contributes to the reduction in Tg. In terms of ion conductivity, the oxygen bridges in VIGLAS materials shorten the distance between Li-Li pairs, thereby facilitating Li+ ion hopping. Additionally, VIGLAS electrolytes exhibit chain segment motion similar to that observed in PEO polymer electrolytes. This motion promotes collective Li+ ions migration in the vicinity, thereby enhancing ionic conductivity.
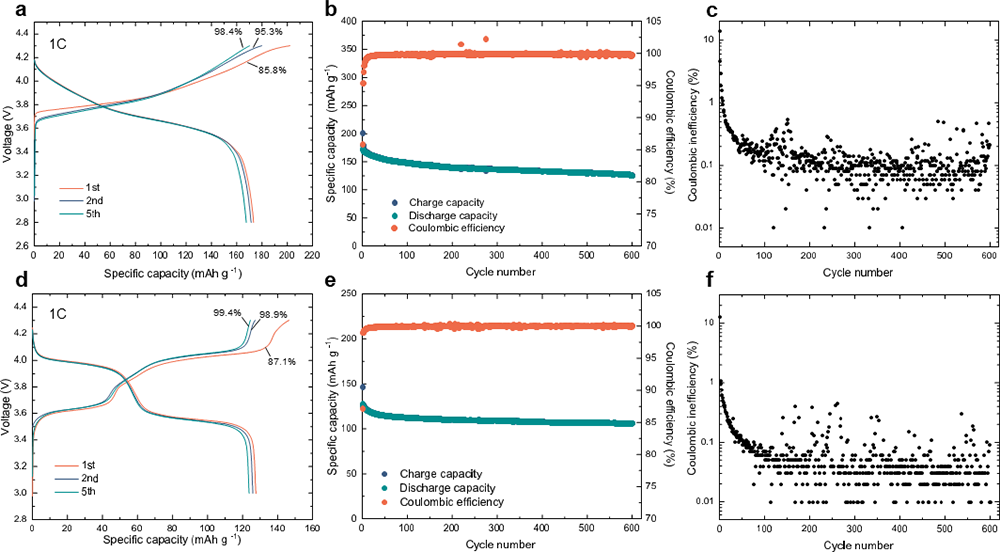
Importantly, the VIGLAS not only exhibit remarkable deformability comparable to organic polymers, but also inherit the desirable traits of conventional inorganic electrolytes. These traits include a high voltage tolerance of up to 4.3 V and impressive ionic conductivity of more than 1 mS/cm. This inherent advantage effectively solves the challenges of mechanical and chemical stability at the positive electrode interface in solid-state batteries. As a result, it enables the groundbreaking achievement of true ambient temperature operation in inorganic all-solid-state lithium and sodium batteries without the need for external pressure (maintained at
Currently, the commercialization of solid-state batteries faces significant challenges associated with high manufacturing costs and complex production processes. Fortunately, this study presents an ideal solution. First, the production cost of this innovative solid-state electrolyte material is exceptionally low, primarily because its core component aluminum, which is abundant in the Earth's crust. This results in a material cost of only $6.85 per kilogram for LACO and only $1.95 per kilogram for NACO. At 2% and 0.6%, respectively, these costs represent a fraction of the current mainstream Li6PS5Cl solid-state electrolyte, which has a high price tag of $319 per kilogram.
Another advantage is that these VIGLAS have a low melting point, below 160 ℃, allowing them to effectively infiltrate porous electrodes like a liquid under suitable heating conditions. This capability makes it possible to achieve commercial cathode loadings in excess of 20 mg/cm2.
Furthermore, these materials exhibit ductility similar to organic polymers, enabling the manufacture of large-area electrolyte films using techniques such as roll-to-roll processes. These remarkable characteristics make this novel solid-state electrolyte material highly competitive in terms of both material and manufacturing costs, making it an ideal choice to address the challenge of pressure-free operation in all-solid-state battery cathodes.
This work was supported by the National Natural Science Foundation of China and the Youth Innovation Promotion Association of CAS.