
This year, Rockefeller scientists illuminated the mysteries of memory and immune evasion. They unraveled how cancer infiltrates the body, how vitamin A influences wound healing, and how the impulse to eat is driven by three neurons. Groundbreaking discoveries in neuroscience, CRISPR technology, and stem cell biology reflect an impressive year for advancement in biomedicine. Here are some of the intriguing discoveries that came out of Rockefeller in 2024.
It turns out Vitamin A is playing an unexpected role in wound repair: hair follicle stem cells are involved in the healing process in a way mediated by retinoic acid, the active form of Vitamin A. A study from the laboratory of Elaine Fuchs found that, during this process, stem cells enter a temporary state of plasticity in which they adopt traits of multiple cell types. Given that an unchecked version of this kind of plasticity is present in some cancer stem cells, the findings raise the possibility that retinoids could also play a role in cancer suppression.
"This hasn't been on the radar until now," Fuchs says. "It's an exciting front to now investigate."
Robert B. Darnell's lab unveiled a tool edging neuroscience much closer to understanding a phenomenon known as dendritic translation-a "holy grail for understanding memory formation," he says. Dendritic translation is a coordinated burst of neuronal activity, which involves an uptick in localized protein production within the spiny branches that project off the neuron cell body and receive signals from other neurons at synapses. It's a process key to memory-and its dysfunction is linked to intellectual disorders.
Using a new platform they developed, Darnell's team identified several previously unknown regulatory mechanisms that drive dendritic translation. The work "defines a whole new biochemical pathway which fits with, complements, and vastly expands what we already knew about memory and learning," Darnell says.

Purkinje cells in the cerebellum, stained and magnified 63 times, revealing fine details of the dendritic spines.
Autism spectrum disorder is tied to more than 70 genes, and the laboratory of Mary E. Hatten has long focused on the link between the ASTN2 gene and cerebellar changes in children with autism, as well as other neurodevelopmental conditions. This year, however, Hatten's lab took that work to the next level with research demonstrating that knocking out ASTN2 in mice induces hallmark autism-like behaviors, including reduced vocalization, diminished social interaction, increased hyperactivity, and repetitive actions.
"It's a big finding in the field of neuroscience," Hatten says, and one that is inspiring her team to delve deeper into the hereditary components of autism. "We're very excited that we've been able to show in detail what ASTN2 does, but there are a lot more genes to investigate."
Metastasis's surprising origins
A pair of compelling studies from the Tavazoie lab challenged conventional wisdom around cancer metastasis. Earlier this year, the lab demonstrated that sensory neurons play a direct role in promoting the growth and spread of breast cancer, providing the first ever evidence of peripheral nerves releasing a signal to enhance metastasis.
Most recently, Tavazoie's team demonstrated, for the first time, that breast cancer metastasis may be, in part, a hereditary disorder driven by mutations in an inherited gene variant common to about 70 percent of white women. The scientists note their finding isn't cause for alarm-the variant does not make people more likely to develop cancer, though it may up the risk for metastasis outcomes by between 2 to 22 percent-but it could open up a new pathway to treatment.
"We've been so focused on the cancer cells, the 'seeds,' that we've ignored the germline-the 'soil'," Tavazoie explains. "It's now clear that focusing on the soil is critical."
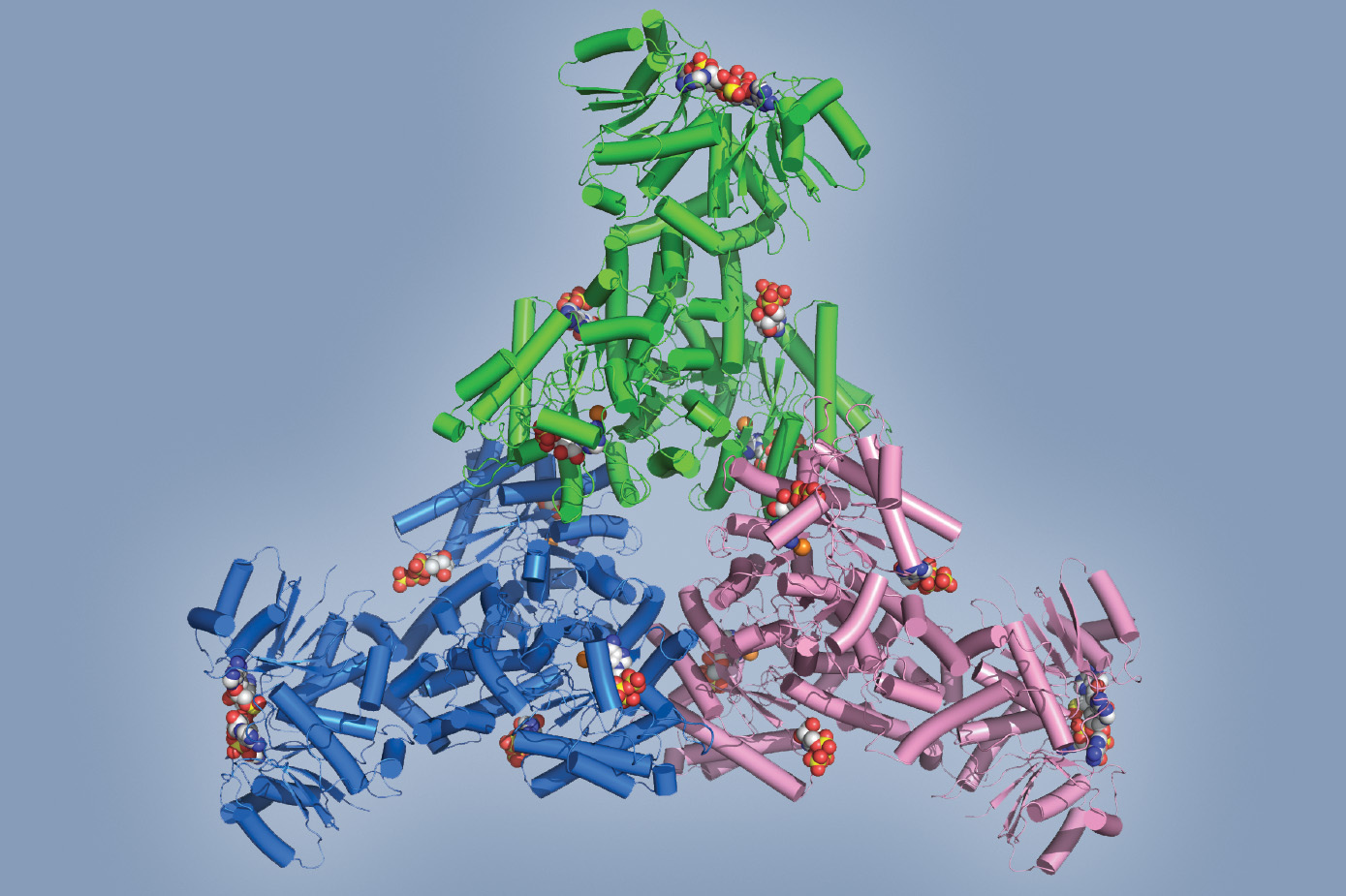
Multicolored model of a hexameric Cad1 protein
CRISPR-Cas9 has long been likened to a kind of genetic scissors, thanks to its ability to snip out any desired section of DNA with elegant precision. Now, thanks to work done in the lab of Luciano Marraffini, we know that certain CRISPR systems operate something like a molecular fumigator as well: In a collaboration with MSKCC, the team found a CRISPR system that also produces a flood of toxic molecules in response invading phages or plasmids, effectively shutting down the infected cell in order to prevent the contagion from spreading through the rest of the bacterial population.
"It's a completely brand-new type of CRISPR chemistry," says Christian Baca, a graduate student in the Marraffini lab. The group envisions one possible application of their discovery may be a diagnostic tool for infection-but it hints at much more. "It's more evidence that CRISPR systems have an array of immune strategies at their disposal."
Telomeres, the protective caps found at chromosome ends, must be kept at just the right length: too short, and dangerously accelerated aging ensues; too long, and they predispose their owners to cancer. Precise regulation is paramount, and Titia de Lange's laboratory recently published work that fundamentally changes our understanding of telomere biology.
Among their findings, de Lange's team discovered that two enzymes work in concert to ensure proper telomere maintenance. Scientists knew the telomerase enzyme helps prevent telomeres from growing shorter with each round of replication. But every DNA double helix has two strands to copy, and telomerase is capable of handling only one. Her team demonstrated how a second enzyme-the CST-Polα-primase complex-manages the other strand.






