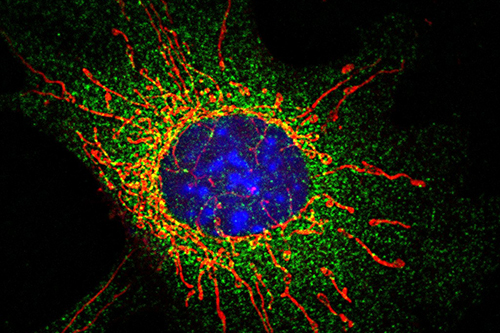
Mitochondria in a fibroblast cell lacking the MFF protein. The mitochondria are elongated due to lack of division. Mitochondria are in red, DRP1 is in green, the nucleus is in blue.University of Bristol
A key molecular step required for the division of damaged mitochondria - essential for cell health - has been identified by a University of Bristol-led study. The finding has the potential to establish how mitochondrial dysfunction goes wrong in common neurodegenerative diseases, such as Parkinson's and Alzheimer's.
The research is published today [4 October] in the journal, Science Advances.
The main function of mitochondria is to generate the energy necessary to power cells. Present in nearly all types of human cell, mitochondria are vital to human survival. Mitochondria are also involved in other tasks, such as signalling, regulation of cellular metabolism, and cell death.
Mitochondrial fission factor (MFF) works by recruiting a secondary protein (DRP1) to the mitochondria to encourage division. However, the molecular details of how this happens are not fully understood.
The research team has identified a key protein in mitochondrial division, mitochondrial fission factor (MFF), as a target of a specific type of modification the lab specialises in called small ubiquitin-like modifier (SUMO).
By combining findings from previous studies, the researchers have demonstrated that MFF, when modified by SUMO, does not act alone to promote division, but exists with inhibitor proteins that bind together. When the mitochondria are damaged, SUMO modifies MFF, which removes these inhibitory proteins, and MFF can then bind to DRP1 to help division.
When MFF cannot be SUMO-modified, the mitochondria do not divide when damaged. This is a key finding and gives the research team a greater understanding of the nuanced rules of mitochondrial division when mitochondria are damaged.
Dr Richard Seager, Research Associate in the School of Biochemistry at the University of Bristol, and first author on the paper, said: "When we realised the previous model of how MFF and DRP1 support division did not fully agree with our findings, we investigated the mechanism in a different way, by looking at other protein players. This revealed a more complex pathway which brought together several previous models of how mitochondrial division works into one model, which is a very exciting finding."
Jeremy Henley, Professor of Molecular Neuroscience in the School of Biochemistry, and corresponding author, added: "Mitochondrial dynamics and the correct regulation of fusion and division are critical for cell health. This is highlighted by the fact that a number of human neurodegenerative diseases are a result of disruption in mitochondrial dynamics due to mutations in the proteins that perform fusion and division.
"More common neurodegenerative diseases, such as Parkinson's and Alzheimer's, show mitochondrial dysfunction, and often more fragmented mitochondria. Understanding the molecular details of mitochondrial division will provide researchers with the potential to establish how this might go wrong and help to possibly prevent and treat diseases."
The research mainly used non-neuronal cells, such as immortalised fibroblasts, which revealed the molecular details of MFF-SUMO modification. Mitochondrial dysfunction is a hallmark of neurodegenerative diseases.
The next step for the research team is to investigate MFF-SUMO in a neuronal context, and examine the effects on mitochondrial morphology and function in neurons, and the resulting effects on neuronal behaviour, such as the connections between neurons, which are very energy demanding structures, and rely heavily on healthy mitochondria.
Paper
'SUMOylation of MFF coordinates fission complexes to promote stress-induced mitochondrial fragmentation' by Richard Seager, Nitheyaa Shree Ramesh, Kevin A. Wilkinson and Jeremy M. Henley et al. in Science Advances






