New research shows how cancer develops in children who are predisposed to Wilms' tumor. This could help to predict the development of tumors before they fully form or to develop new, targeted therapies.
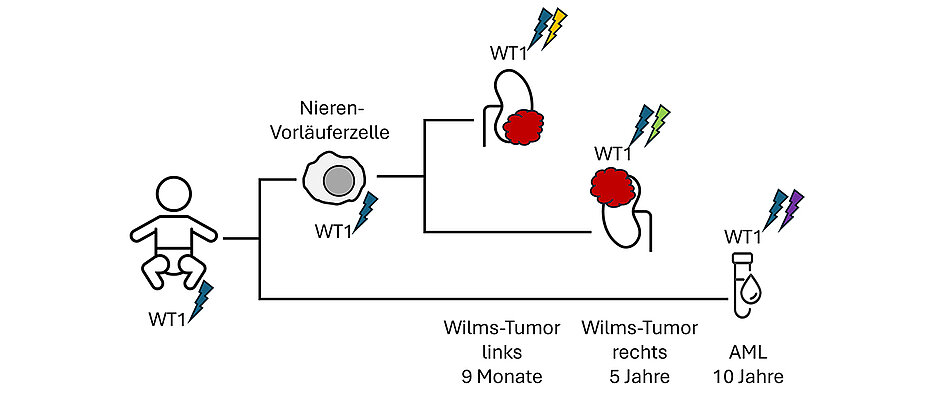
Wilms' tumor is a form of kidney cancer that mainly affects children under the age of five. In Germany, around 100 children are diagnosed with it every year. These tumors usually develop as a result of a spontaneous genetic change during development in the womb. In some cases, however, the risk of developing a Wilms tumor is significantly increased due to a genetic predisposition, a so-called germline mutation.
Traditionally, children with Wilms' tumor are examined for such a predisposition if they have certain characteristics, such as typical accompanying malformations or bilateral tumors. However, many cases have apparently been overlooked, so that it is likely that far more than the ten percent of affected children suspected to date have such a predisposition.
Tissue samples from 137 children examined
In a new study now published in the journal Cancer Discovery, scientists have analyzed the genetic differences in children with Wilms' tumor. They genetically mapped several hundred tissue samples from 137 children with this type of tumor, including 71 children with a genetic predisposition, some of whom showed early symptoms.
A research team from the Department of Developmental Biochemistry at the Biocenter of Julius-Maximilians-Universität Würzburg (JMU) was involved in the study, together with partners from the Wellcome Sanger Institute, Cambridge University Hospitals NHS Foundation Trust and Great Ormond Street Hospital in London.
Certain mutations increase the risk of further tumors
It was found that various types of genetic predisposition and sometimes complex structural changes in chromosomes can cause tumor formation. Some of these so-called driver mutations also increase the risk of children to develop secondary cancers, in some cases not until later in adulthood. Tumors with changes in the WT1 gene formed a relatively large subgroup of their own, while the other tumors exhibited a wide variety of other and in some cases previously unknown driver mutations. Such findings could also be targeted in the future development of drugs.
This subdivision of tumors into genetically defined subgroups was also evident in the analysis of gene expression patterns and DNA methylation. If several tissue samples or later recurrent tumors could be analyzed, genome sequencing could even be used to trace the presumed origin of the first tumor and the sequence of steps leading up to recurrence.
Tailor-made treatment plans for affected children
One aim of the work is to be able to guarantee the most effective treatment for affected children in future with genetically tailored treatment plans, while at the same time minimizing side effects as far as possible. This is based on the knowledge that the inherited genetic changes determine how these tumors develop, how strongly they respond to certain treatments and whether those affected have a higher risk of developing other types of cancer later in life.
The clinical treatment of children with a known hereditary predisposition differs from the treatment of children with a spontaneous genetic change due to the increased risk. Knowledge of such a predisposition therefore not only determines the treatment strategy and the risk of recurrence, but also whether siblings or later own children are exposed to such an increased risk, or even carry a risk of further tumor types.
Basis for an adapted therapy
"This work represents an important step towards determining the frequency of a genetic predisposition to Wilms' tumour and paves the way for optimized and personalized treatment of these children and their families," comments Professor Manfred Gessler on the study. The biochemist holds the Chair of Developmental Biochemistry at JMU and led the research together with Professor Sam Behjati from the Wellcome Sanger Institute and Cambridge University Hospitals NHS Foundation Trust.
Dr. Taryn Treger, co-first author at the Wellcome Sanger Institute, says: "Our research shows that cancers develop in different ways, depending on what the underlying genetic change is. This means that in some predispositions we can exactly predict what additional genetic changes lead to cancer development, paving the path to identify treatments that interfere with cancer formation in the first place." Dr. Jenny Wegert, co-first author from Würzburg, adds: "The study not only lays the groundwork for improved screening programs and risk assessment for affected children. There is also the hope of being able to significantly reduce the risk of late effects through adapted therapeutic approaches in surgery and chemotherapy".
Funding
This research was funded in part by the Little Princess Trust and Wellcome and support for the German Wilms-Tumor Biobank through DFG and BMBF.
Publication
T. D. Treger, J. Wegert, A. Wenger, et al. (2025) Predisposition footprints in the somatic genome of Wilms tumours. Cancer Discovery. DOI: 10.1158/2159-8290.CD-24-0878






