Scientists at King's College London and UCL in collaboration with Moderna Inc. have exploited the same mRNA technology used in Covid-19 vaccines, to create an effective therapy for a rare disease in mice - demonstrating the technology's potential use against rare diseases in people.
Research, published in Science Translational Medicine, has found that messenger RNA (mRNA) could be used to correct a model of a rare liver genetic disease known as argininosuccinic aciduria.
Argininosuccinic aciduria is an inherited metabolic disorder that occurs in approximately one in 100,000 newborns and affects how the body breaks down protein - potentially leading to high levels of ammonia.
Patients affected by the disease were found to also experience an imbalance glutathione regulation, which is important for liver detoxification.
This is great example of collaborative science across multiple areas of expertise, which has yielded remarkable results. By understanding what goes wrong in this disease, we can not only correct the error, but follow this correction in real-time using imaging. We are looking forward to bring these advances to patients in the near-future.
Dr Tim Witney, Co-Lead PI, Reader in Molecular Imaging at the School of Biomedical Engineering & Imaging Sciences
Messenger RNA (mRNA) has revolutionised the field of vaccines during the COVID-19 pandemic. We believe it can now do the same for rare diseases.We have shown that mRNA holds an unprecedented therapeutic potential for incurable genetic diseases, in particular liver conditions. We aim to apply this approach to other liver inherited diseases and translate mRNA therapy to patients, especially in children.
Dr Julien Baruteau, Co-lead PI, UCL Great Ormond Street Institute of Child Health
Rare diseases usually result from errors in the patient's DNA and affect around 300 million people worldwide.
However, fewer than 5% of these conditions have approved therapies. Most of these treatments use gene therapy to switch out the faulty gene and replace it with a normal functioning one, to alleviate the disease.
Until recently, gene therapy employed modified viruses to bring the therapeutic gene to the disease cells. However, these viral systems can cause severe adverse effects, such as reactions from the patient's own immune system, meaning that they can't be rolled out widely.
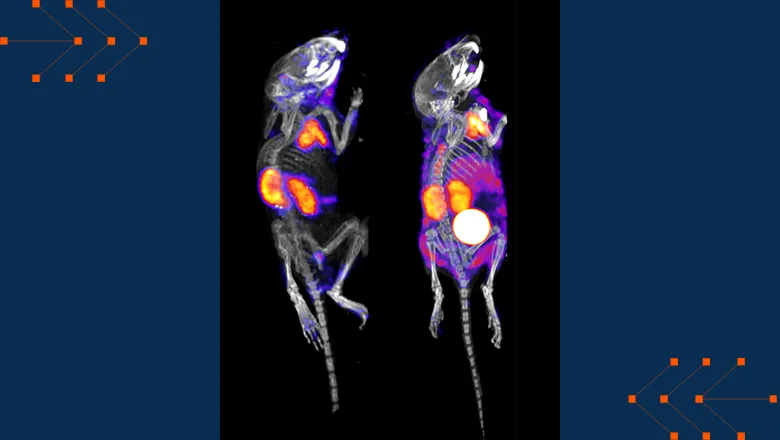
Consequently, the team wanted to investigate the possibility of using mRNA technology as an alternative solution. Over the course of the trial, mice were given positron emission tomography (PET) scans as a non-invasive way to track the correction of glutathione regulation and the success of the treatment.
Over the coming years, the team aims to trial the therapy in humans, and is currently testing this technology on other rare diseases - propionic and methylmalonic acidaemias - in clinical trials sponsored by Moderna Inc. at Great Ormond Street Hospital for Children.
The research was funded by Moderna Inc., the Medical Research Council, the consortium London Advanced Therapies, The Wellcome Trust, CRUK, and the National Institute for Health and Care Research (NIHR) Great Ormond Street Hospital Biomedical Research Centre.
Dr Paolo Martini, Chief Scientific Officer for International Therapeutics Research Centres at Moderna, said: "This collaboration has exemplified how academia and industry can work in synergy to explore how mRNA technology can be harnessed against rare diseases and may potentially lead to a treatment for a severe and debilitating disease such as argininosuccinic aciduria."






