The development of new antibiotics has stalled - new strategies are needed as the world enters the age of antibiotic resistance.
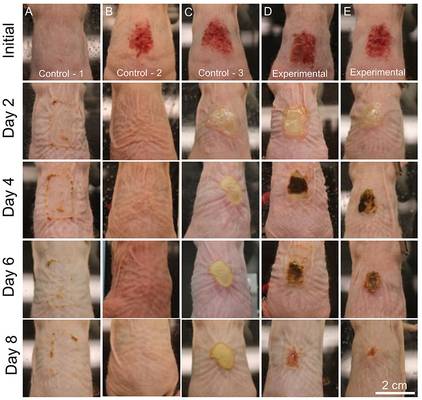
To combat this challenge, Lawrence Livermore National Laboratory (LLNL) scientists have found that synthetic antibacterial minerals exhibit potent antibacterial activity against topical MRSA infections and increase the rate of wound closure.
Methicillin-resistant Staphylococcus aureus (MRSA) infection is caused by a type of staph bacteria that's become resistant to many of the antibiotics used to treat ordinary staph infections.
Previous research showed that naturally occurring clays have been effective in killing antibiotic resistant bacteria. However, these natural clays are too variable to be used in clinical settings.
"Our research shows that synthetic antibacterial minerals can successfully eliminate MRSA biofilms in topical wounds and increase wound closure rates," said LLNL scientist Keith Morrison, lead author of a paper appearing in Nature. "The exploration of synergistic mineral-based geochemical reactions for antimicrobial applications is still in its infancy and further research is needed to transition this technology into clinical settings."
Metals have been used throughout history to combat microbial infections, with the application of silver and copper receiving the most attention. Iron has been shown to limit bacterial growth but can generate toxicity if excess uptake occurs. Excess iron in wounds is traditionally viewed as a negative outcome for wound healing, as it accelerates bacterial growth and the formation of biofilm infections while promoting toxicity from reactive oxygen species (ROS) generation. The chelation of excess iron in wounds has been used as a strategy to limit bacterial growth and promote wound healing, but success with this approach has been limited.
The discovery of natural clay mineral deposits with iron-based antibacterial activity revealed that geochemical reactions that maintain ferrous iron and ROS can serve as potent antimicrobials.These antibacterial zones in natural deposits are composed of smectite clays and iron sulfides (pyrite) that experience a cascade of redox reactions as they achieve a new chemical equilibrium. This strategy of 'mineral iron-overload' to cure antibiotic-resistant infections appears counterintuitive when compared to the common assumptions that excess iron and ROS are associated with delayed healing and negative outcomes in chronic wounds. However, anecdotal evidence from natural clays and limited animal-model MRSA infections reveals that these antibacterial mineral mixtures may promote wound healing while killing antibiotic resistant infections. The clinical application of these natural mineral deposits has proved difficult due to the large variations in mineralogy and antibacterial activity, along with the presence of toxic metal impurities that produce variable outcomes in wound care.
The recent synthesis of antibacterial minerals with properties that mimic the natural minerals proved successful at killing the ESKAPE pathogens (Enterococcus sp., Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter sp., Pseudomonas aeruginosa and Enterobacter sp.), providing a chemically pure and consistent dose that can be scaled for pharmaceutical production.
The synthetic antibacterial mineral systems also can be tuned to have different reaction rates that can maintain ferrous iron and ROS production at specific concentrations from days to weeks when hydrated. This tuning and control of concentration and reactivity is not possible with the application of metal solutions alone. Materials that control the solubility and speciation of metal solutions are needed to make a clinically effective metal-based antimicrobial.
"These results provide evidence for the strategy of 'iron overload' to combat antibiotic-resistant infections through the maintained release of iron and generation of ROS via distinct geochemical reactions that can break the chronic wound damage cycle," Morrison said. "These minerals may be compatible with a variety of self-assembled nanomaterials, polymers and 3D-printed wound dressings that can be used to control and tune novel antibacterial mechanisms."
A patent has been filed on this technology and Morrison's research team is looking for industry partners to help commercialize this novel antimicrobial approach.
Other LLNL researchers include Meghan Reiss, Tanya Tanner, Travis Gollott, Nicole Collette and Gabriela Loots, former LLNL scientist now at UC Merced. The work was funded by LLNL's Laboratory Directed Research and Development program.