
Cell-to-cell communication through nanosized particles, working as messengers and carriers, can now be analyzed in a whole new way, thanks to a new method involving CRISPR gene-editing technology. The particles, known as small extracellular vesicles (sEVs), play an important role in the spread of disease and as potential drug carriers. The newly developed system, named CIBER, enables thousands of genes to be studied at once, by labeling sEVs with a kind of RNA "barcode." With this, researchers hope to find what factors are involved in sEV release from host cells. This will help advance our understanding of basic sEV biology and may aid in the development of new treatments for diseases, such as cancer.
Your body "talks" in more ways than one. Your cells communicate with each other, enabling your different parts to function as one team. However, there are still many mysteries surrounding this process. Extracellular vesicles (EVs), small particles released by cells, were previously thought to be useless waste. However, in recent decades they have been dramatically relabeled as very important particles (VIPs), due to their association with various diseases, including cancer, neurodegenerative diseases and age-related diseases.
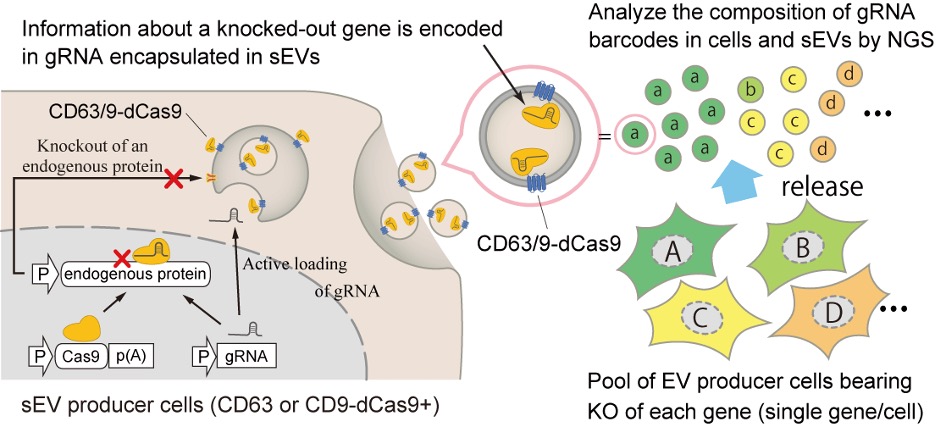
Small EVs have been found to play a key role in cell-to-cell communication. Depending on what "cargo" they carry from their host cell (which can include RNA, proteins and lipids), sEVs can help maintain normal tissue functions or can further the spread of diseases. Because of this, researchers are interested in how sEVs form and are released. However, separating sEVs from other molecules and identifying the factors which lead to their release is both difficult and time-consuming with conventional methods. So, a team in Japan has developed a new technique.
"We have established a new high-throughput screening platform named CIBER (CRISPR-assisted individually barcoded sEV-based release regulator). This system allows a single experimenter to implement a genome-wide screening of sEV release regulators within several weeks to a couple of months, which is superefficient compared to the conventional methods," explained Associate Professor Ryosuke Kojima from the Graduate School of Medicine at the University of Tokyo. "CIBER should be a valuable tool for detailed studies on the creation, release and diversity of sEVs."
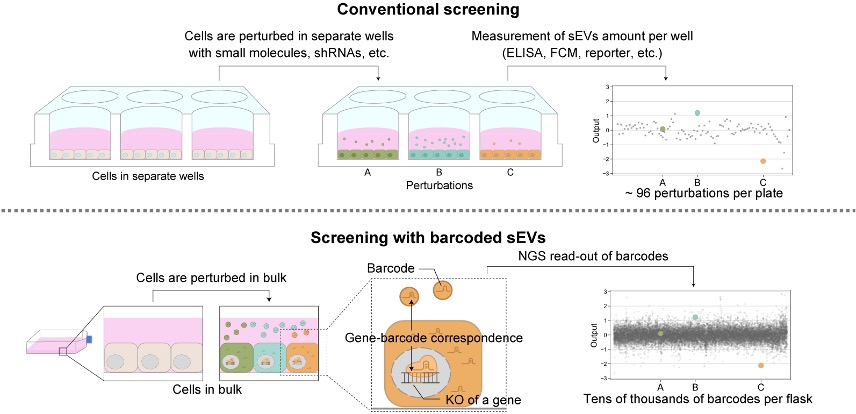
The CIBER system works by using CRISPR-guide RNA (gRNA) to knock out (incapacitate) a specific gene in each cell, which in turn is barcoded into the sEVs the cell releases. This enables the researchers to track and estimate the amount of sEVs released by the host cell. Current methods to study factors involved in sEV release involve separating cells into wells, disturbing the expression and activity of a gene in the cells in each well, and then measuring how that impacts the amount of sEVs released. However, with the CIBER system, thousands of cells with different genes knocked out can be studied together in one pool. Thanks to this, researchers can study the various complex factors involved in sEV release simultaneously, as well as estimate the amount of sEVs and different types (subpopulations) released from each cell.
"In the future, CIBER screening could be used to identify the therapeutic target of sEV releases or enhance the production of sEVs for therapeutic use, such as for cancer," said Kojima. "Barcoded sEVs could also be used to estimate cell population dynamics without destroying cells, and tracing the fate of barcoded sEVs can help us better understand sEV biology. We believe that CIBER screening has great potential."






