Lysosomes play an important role in cells and tissues, controlling not only the degradation of substances but also cell division and growth.
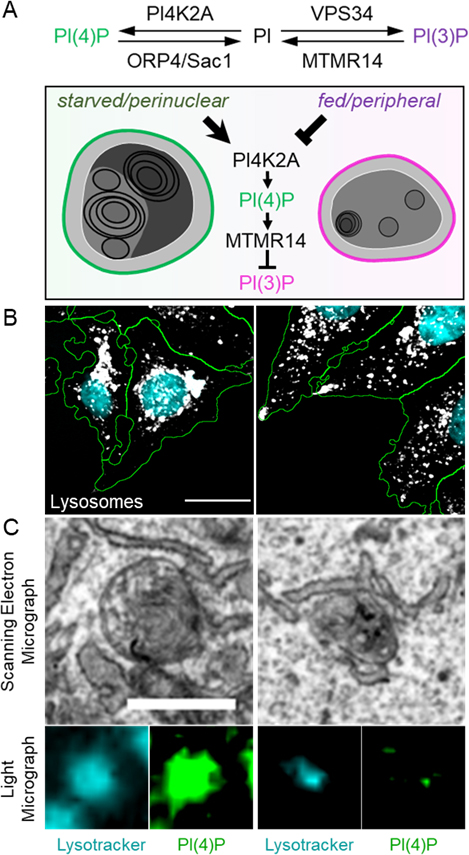
A) The nutrient-dependent transformation of lysosomes is controlled by enzymes that can interconvert the signaling lipids PI(4)P and PI(3)P. B) In starvation (left) and nutrient-rich conditions (right), lysosomes display different subcellular localization patterns. C) Imaging techniques that can combine electron and light microscopy reveal the ultrastructure of lysosomes (marked with lysotracker) with different levels of specific signaling lipids (PI(4)P in green). | © Michael Ebner
A team led by Professor Volker Haucke and Dr. Michael Ebner at the Leibniz-Forschungsinstitut für Molekulare Pharmakologie (FMP) has investigated how these two functions are related to nutrient availability in the cell. The researchers were able to show for the first time that lysosomes undergo a massive transformation. A signaling lipid acts as a switch between the two states. The findings, published in the journal Cell, could be used to develop drugs that specifically stimulate cells from patients with neurodegenerative or metabolic diseases to break down harmful protein molecules inside the cell.
Nutrient availability in the body is constantly changing. For example, after a full meal, cells have many more nutrients available than at the end of a long night without any food intake. "It is important for all cells and tissues to be able to respond to the current food intake in such a way that certain basic building blocks are always present inside the cell," explained Professor Volker Haucke, Director of the Leibniz-Forschungsinstitut für Molekulare Pharmakologie (FMP). These basic building blocks are obtained through catabolism, the process by which ingested nutrients are broken down into small components that the cell uses to make the molecules it needs.
One of the components responsible for this is the lysosome, a membrane-enclosed sac. At the same time, lysosomes are the central monitoring point that determines whether the food supply in the cell is good or bad. When there is a good supply of nutrients, the mTOR signaling pathway on lysosomes is activated, inducing cell division and growth. When nutrients are scarce, the mTOR complex is switched off to stimulate catabolic programs. As a result, lysosomes combine two opposing tasks: degradation and assembly. "Until now, it was not known whether there are different types of lysosomes in the cell or whether they change. This was the fundamental question we addressed in our study," stated Michael Ebner, cell biologist at the FMP and lead author of the study.
Using a light microscope, the researchers analyzed the behavior of lysosomes in cells that were switched from fed to starved state within one to two hours. This made it possible to observe the fluorescence-labeled organelles in detail and in 3D. The team also developed biochemical methods to characterize lysosomes in both states. "We were able to see that the cell undergoes a drastic transformation when the food supply changes," reported Volker Haucke. In a complex cascade, this process is controlled by signaling lipid molecules that induce either a starved or a fed state. Using correlative light and electron microscopy, the researchers observed that there are two pools of lysosomes in the cell: Small motile ones, located more at the periphery, act as monitoring stations. Meanwhile, larger, more static lysosomes, located closer to the nucleus, are responsible for degradation. What changes is the ratio: In a well-fed state, the small motile lysosomes carrying the active mTOR complex predominate, and there are relatively few static lysosomes. When the cell starves, the small motile lysosomes lose the signaling lipid marker for mTOR and acquire a new signaling lipid, activating the digestive enzymes in the lysosome. "This response is acute, meaning that the cells are transformed immediately, and initial changes can be observed within minutes. The process from destructive to constructive metabolism is completed in one to two hours," reported Michael Ebner.
The signaling lipids act as a switch that activates or switches off the mTOR complex, depending on nutrient availability. "Lysosome properties change completely, depending on the signaling lipid," emphasized Volker Haucke. This makes the new findings interesting for therapeutic purposes. After all, during active degradation, lysosomes also remove damaged proteins. And if you can flip the signaling lipid switch artificially, you can also influence the metabolic events in the cell. This phenomenon may be exploited to treat diseases such as Alzheimer's, a neurodegenerative disease characterized by the accumulation of defective proteins in the cell. "By flipping the lipid switch to starvation, you activate exactly this type of degradation in the cells without changing anything else. We can therefore intervene in cellular metabolism in a new way with a rather simple switch," remarked Volker Haucke.
Next, the researchers want to find suitable compounds, i.e. small molecules that are able to flip the corresponding signaling lipid switch. For another study, the FMP has partnered with the German Institute of Human Nutrition (Dife) and the Leibniz-Institut für Analytische Wissenschaften (ISAS). Using new analytical methods, data from obese patients, and studies in animal models, the researchers aim to identify suitable therapeutic targets.
Michael Ebner, Dmytro Puchkov, Orestes Lopez Ortega, Pathma Muthukottiappan, Yanwei Su, Christopher Schmied, Silke Zillmann, Iryna Nikonenko, Jochen Koddebusch, Gillian L. Dornan, Max T. Lucht, Vonda Koka, Wonyul Jang, Philipp Alexander Koch, Alexander Wallroth, Martin Lehmann, Britta Brügger, Mario Pende, Dominic Winter, Volker Haucke (2023)
Nutrient-regulated control of lysosome function by signaling lipid conversion.
Cell online. DOI: 10.1016/j.cell.2023.09.027






