Electrons hold the world together. When chemical reactions yield new substances, they play a leading role. And in electronics, too, they are the protagonists. Together with his colleagues, Ferenc Krausz, Director of the Max Planck Institute of Quantum Optics in Garching, photographs the rapid movements of electrons with attosecond flashes, creating the basis for new technological developments.

The long path for attopulses: Manish Garg (left) and Antoine Moulet work at the experiment bench over which the attosecond pulses are directed. Because the photo was taken with an extremely wide-angle lens, the straight bench appears curved.
© Thorsten Naeser
The black curtain goes up: As if on a stage, below us lies a cleanroom, about the size of a school gymnasium. It is almost entirely filled with a laser system. Here, powerful laser beams zip through the air, containing a stream of femtosecond light pulses lasting just a few millionths of a billionth of a second. The system is so sensitive that we can only admire it through an observation window. The complex assembly of optical instruments before us forms the first section of a speedway where the world's shortest flashes of light cross the finish line. But this is no racetrack: we're in the department of Ferenc Krausz at the Max Planck Institute of Quantum Optics in Garching.
The femtosecond laser pulses - which are already very short - travel through a vacuum tube to a lab one floor down. We're allowed to enter this lab. This is where attosecond flashes are created, which are a thousand times shorter than femtopulses. In the lab stands a large barrel to which doctoral students Martin Schäffer and Johann Riemensberger are making some adjustments. The barrel is vaguely reminiscent of an enormous washing machine drum crossed with an old diving helmet. In reality, it's a vacuum chamber made of solid stainless steel. Its armored-glass windows permit a glimpse of the probe that marks the target of the attosecond flashes of light.
Schäffer and Riemensberger belong to the Garching-based team of Reinhard Kienberger, who is also a physics professor at Technische Universität München (TUM). More than a hundred scientists conduct their research in Ferenc Krausz's department. The pioneer of attosecond technology attracts young scientists from all over the world who want to observe some of nature's extremely fast processes.
Krausz is therefore the perfect person to convey a sense of how fast an attosecond is. Purely mathematically, an attosecond is a billionth of a billionth of a second. Even as a physicist, Krausz finds this numbers game lacking in clarity. So he searches for suitable comparisons. "The fastest thing we know is light," he explains, "which can circle the earth approximately eight times in one second." But even though light is so fast, within one attosecond, it could only travel from one end of a single water molecule to the other!" With a diameter of only 0.3 nanometres (a nanometre is one millionth of a millimetre), a water molecule is unimaginably tiny. "Nano" is derived from the Ancient Greek word for dwarf, nanos, and that is precisely what attosecond physics is about: extremely dwarfish quantum objects that move incredibly quickly.
A high-speed camera for electrons
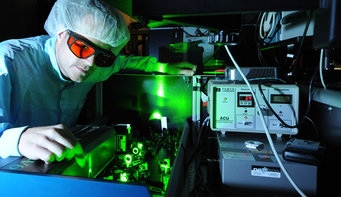
Clean air for intense laser light: Tim Paasch-Colberg is working on the laser system in the cleanroom, where intense femtosecond pulses are produced. Any dust in the air could destroy the delicate optics.
© Thorsten Naeser
Anyone who wants to understand the motivation of scientists like Ferenc Krausz in a historical context should consider the horse. For centuries, it was disputed whether all four of a horse's hooves left the ground when galloping. Generations of painters devoted themselves to this topic. Eadweard Muybridge solved the mystery: yes, for a brief moment, all four hooves are in the air. In 1878, Muybridge succeeded for the first time in freezing all phases of a horse's gallop in rapid snapshots.
Since then, high-speed images of processes that happen faster than the eye can see have expanded our knowledge considerably. In natural sciences, the decryption of ultrafast processes in the world of atoms and elementary particles is even essential.
Attosecond research in Garching focuses on electrons. "These are practically everywhere," comments Krausz on the predominance of this negatively charged elementary particle. Although electrons are extremely small, even from the perspective of the nanoworld, they are the quantum glue that joins atoms together to construct the diverse forms of matter that make up our world. Moreover, electrons play a leading role in chemical reactions. New substances are always created by electrons being pushed in between different atoms. Until recently, though, it wasn't possible to trace the activity of these elementary particles directly due to the speed at which they act.
In other words, for researchers, electrons had long behaved like shellgame tricksters, fooling onlookers with their speed. Quantum mechanics can describe their behaviour theoretically, but primarily just the initial and final situations in these electronic shell games. This was long the case for experiments, too: the electrons' actual motion remained hidden. Not only was this gap in knowledge unsatisfactory from the point of view of basic research, but even today, it also impedes new, practical developments. Take catalysts, for example, which make chemical reaction processes more efficient. In order to systematically identify the best material to provide chemical assistance, researchers must be able to understand just how a catalyst influences electron transfer.
Live images of chemical reactions
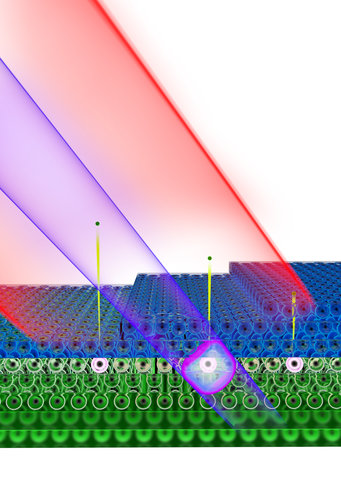
Electrons passing through: Attosecond pulses from extremely ultraviolet light (violet) catapult electrons out of the atoms of a tungsten crystal (green). Using infrared laser pulses (red), the researchers in Garching measured that an electron takes 40 attoseconds to pass through a single layer of magnesium atoms (blue) above the tungsten crystal.
© Christian Hackenberger/Nature 2015
As lasers became more and more powerful in the 1970s, a fascinating idea arose: perhaps extremely short laser pulses could be used to take snapshots of chemical reactions, as Muybridge once photographed the phases of the horse gallop. "Individual images taken at different times can then be used to create a film that shows the precise reaction process," explains Krausz. This approach created a new field of research based on what is known as the pump-probe method. In this method, a first laser flash initiates a process, such as a chemical reaction, and after a precisely determined delay, a second flash takes a snapshot. By repeating the reaction and varying the delay between the pump and the probe laser pulses, the scientists obtain images of every stage of a process. They can then compile these into a film.
By the 1990s, this experimental technique was so sophisticated that it provided completely new insights into nature, making the movement of atoms in molecules visible for the first time. For this pioneering achievement, Egyptian-American physical chemist Ahmed Zewail was awarded the Nobel Prize in Chemistry in 1999.
How do you get laser flashes in the X-ray range?
At the same time, however, what had until then been rapid development toward ever shorter shutter speeds ran up against a wall. Although the technique was capable of showing processes that occur in just a few femtoseconds in slow motion, it was only the movements of atoms that could be filmed, not those of electrons, which are much lighter and faster. In order to freeze the latter in a snapshot, a radical new attosecond flash technology was needed.
Around the turn of the millennium, Ferenc Krausz, then at Technische Universität Wien, was the first to succeed in breaking the barrier to the attosecond range. Since then, there have been steady improvements in filming the movement of electrons, and a new field of research has emerged. The scientists in Garching are now capturing the speedy world of electrons on film. But why did this technological barrier in ultrashort-time research exist?
"It has to do with the colour of the light - in other words, its wavelength," explains Krausz. A light pulse can't travel through space unless it comprises at least one wave crest and one wave trough. Only pulses from such a complete wave train are inherently so stable that they can make their way from the laser to the probe without completely dissipating. However, if a wave train is to be squeezed into an interval of a few attoseconds, its wavelength must be sufficiently short.
"For pulses of less than one femtosecond, extreme ultraviolet laser light is needed," says Krausz, "and to produce even shorter pulses, there's no way around using X-ray light." And that was precisely the problem: for a long time, there were no lasers that could produce light in this shortwave spectral range. Only in recent years have so-called freeelectron lasers made advances into this range. But they have one drawback: they require kilometre-long particle accelerators - enormous, expensive facilities for large-scale research.
Krausz's idea: Noble gas atoms generate ultra-short flashes of lightning
In the 1990s, Krausz - like Muybridge in his time - took on the challenge of clearing this hurdle using existing technology. Back then, Muybridge combined his giant plate cameras into one long row, each being triggered in succession by a horse galloping past.
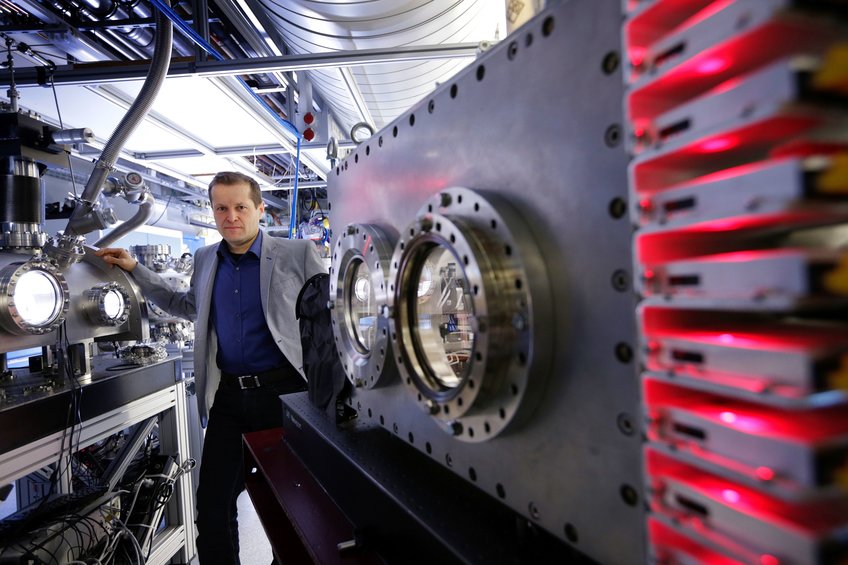
Moviemaker in the quantum world: Ferenc Krausz is a pioneer of attosecond metrology, a technique used to capture the movements of electrons. The foreground and the vacuum chambers to his left serve as filming locations where these experiments take place.
© Jan Greune/LMU
Thus he managed to capture an image of each phase of the gallop.
Krausz had to make do with femtosecond laser technology that had too long a wavelength. He hit upon the idea of shooting the high-intensity laser pulses at noble gas atoms. The femtosecond laser light jolts the atoms and forces them to emit a flash in the ultraviolet or X-ray range. Because the atoms oscillate precisely in time with the excitationfemtosecond laser pulses, this shortwave light happens to have the pure, perfect quality of laser light. This trick allowed Krausz to advance into the attosecond range for the first time.
Since then, the scientists in Garching have continuously improved their attosecond facilities. In 2004, Krausz's group achieved the world record for the shortest light pulse, at 650 attoseconds. Today, the best experiments flash around ten times faster. "With Martin Schultze's team, we here in Garching have pared this down to 72 attoseconds," says Krausz, "but since 2012, Zenghu Chang's group at the University of Central Florida holds the world record, with a mere 67 attoseconds." The Max Planck Director isn't bothered that Garching lost the record - on the contrary. "This shows that our research field is the subject of growing interest," he explains proudly. In the end, it's not about records, but about increasingly refined tools for studying the fastest movements in the microcosm.
Insights into the electron play of crystals
Among the first subjects of attosecond metrology or attosecond chronoscopy, as the specialists in Garching call their technology, were the movements of electrons in individual atoms and molecules. However, for a few years now the researchers have been focusing on far more complex electron worlds: crystalline solids. In many inorganic materials, the atoms form the regular, spatial grid of a crystal. Crystals occur in great variety in nature; even the metals and semiconductors that are so useful for countless applications form crystals.
With the new attosecond measuring technology, the team headed by Reinhard Kienberger has just recorded in real time how electrons zoom through a single layer of metal atoms. This passage is of key importance for both physics and technology. Its direct observation can one day aid in developing substantially faster electronic switching elements and microprocessors.
Basic researchers are interested in the complex behaviour of the electrons in crystals because the elementary particles in them have two very central effects that together determine many properties of matter. For instance, some of the electrons bind atoms together to form a crystal lattice. They essentially make up the quantum glue of matter. The second effect is caused by electrons that can detach from their original atoms. In metals, for example, they whiz more or less freely through the crystal lattice, and can thus transport electric current. In addition, these conduction electrons have a major in- fluence on the mechanical and optical properties of a material, and its ability to conduct heat.
In many crystals, however, there are no conduction electrons. These materials are thus known as insulators, one example of which is quartz. Semiconductors are a sort of hybrid between insulators and electrical conductors. As a material used in electronics construction, semiconductors have radically changed our culture. In these materials, the electrons need a small push to be able to flow as conduction electrons. To understand this behaviour, we must look at electrons as quantum particles.
Light flashes make insulators conductive
Since an electron is also an electromagnetic wave, it has a wavelength, which is linked to its kinetic energy - a bit like the sound of a racecar is linked to the speed of its engine. If an electron wants to move freely through a crystal lattice, its wavelength must match the spatial grid of the atoms. This is applicable for only a certain range of wavelengths - and a certain range of energy. This range forms a kind of highway on which electrons can race through the crystal. In metals, there is always a lot of electron traffic flowing in this conduction band. In semiconductors, however, even the most mobile electrons cling tightly to their atoms. They require an energy kick to manage the quantum leap into the conduction band. This kick is used, for example, to switch operations in transistors.
In insulators, however, this energy kick would have to be so intense that it would rip the material. Could one nevertheless make an insulator conductive, even if only for a short time? And would it also be interesting from a technological standpoint?
These are the questions a Garchingbased team of attosecond researchers, in which Elisabeth Bothschafter and Martin Schultze were doing their doctoral work, asked themselves. To find answers, the laser physicists needed well-versed crystals experts on their side. They found these solid-state physicists in theorist Mark Stockman's group at Georgia State University in Atlanta, USA. This collaboration was facilitated by Augustin Schiffrin, an American who had just relocated to Garching to conduct attosecond research himself.
In the lab in Garching, the research team used quartz to explore whether this insulator can be made conductive for a short time when bombarded with extremely intense femtosecond light flashes - without being destroyed. The researchers were also interested in how the flashes of light influence its optical properties. They tracked this momentary change with even shorter attosecond flashes.
Atto flashes illuminate what is happening in the crystal
To do this, Bothschafter and Schultze developed an experiment in which the experts in Garching reduced their record to a pulse duration of just 72 attoseconds. The researchers sent these attosecond flashes to their probe, synchronized with femtosecond light pulses. This gave them the high-precision tool necessary to scan the exact form of the femtosecond light pulses they used. This was the key to correctly interpreting the change these pulses caused in the quartz.
The physicists used the attopulses to analyse in real time and in detail what happened in the barely 200-nanometer-thick quartz probe when bombarded with light. They found that the intense femtosecond laser pulse, which is an electromagnetic wave, produced an extremely strong electric field in the probe. Martin Schultze compares it with the field of an overhead high-voltage line connected between two electrodes spaced just a few thousandths of a millimetre apart. Every known material would vaporize immediately.
The quartz survived solely because this electric inferno raged inside it for only a few femtoseconds. It was, quite simply, too sluggish to notice it. The ordeal was long since over and done before its atoms could drift apart. For the electrons, however, it was quite a different story. Some of them followed the field of the femtosecond pulse like dogs on a leash. As a result, they temporarily produced an electric current in the quartz that immediately subsided again at the end of the femtosecond pulse. Then the quartz became a normal insulator again.
"This means that, using the light pulse, it is possible not only to turn on conductivity in the inconceivably short span of a few femtoseconds, but also to turn it off again," says Krausz. The latter was crucial, as this doesn't happen in semiconductors.
Stockman's group showed that, in this situation, electrons move according to a completely different mechanism than in semiconductors. In the latter, they can be kicked into the conduction band within femtoseconds, but then, from the perspective of attosecond physics, they stay there for an eternity: namely one thousand to ten thousand times longer than a femtosecond. In quartz, however, the electrons immediately follow the electric light field away from their atoms, and jump back just as quickly as soon as the field fades away. Thus, with this method, the researchers obtained an electric switch that acts extremely rapidly - within femtoseconds.
The physicists in Garching and their collaborative partners initially addressed these processes as basic researchers, and in doing so, they uncovered something completely new: never before has anyone produced such an exotic state in an insulator. In the long term, this discovery could revolutionize electronics, since it is the relatively sluggish persistence of electrons in the conduction band of semiconductors that limits the switching speed of conventional transistors. In the lab, using extremely small nanostructures, conventional transistors already reach speeds of hundreds of billions of cycles per second. That's roughly one order of magnitude faster than established computer microprocessors today.
"Our discovery would make it possible to achieve circuit times that are another ten thousand times faster," says Krausz, summing up. This would allow computers to process vast amounts of data in real time. Of course, this would also require being able to miniaturize the meter-long short-pulse lasers that drive the switching operation. "For that reason - and for a few others, as well - this is currently just an interesting prospect for the future," he emphasizes.
For Krausz, as a Max Planck scientist, the focus is on the knowledge gained. With the new attosecond measuring technology, Reinhard Kienberger's team just recorded in real time how electrons dart through individual atomic layers of a crystal lattice. This "journey" is of key importance for physics and technology. Direct observation can be decisive in helping to one day develop significantly faster electronic switching elements and microprocessors.






