
Illinois professor Brian Cunningham led a team that developed a microscope technique capable of seeing and counting individual viruses that could underpin new rapid diagnostics, including for COVID-19.
Photo by L. Brian Stauffer
CHAMPAIGN, Ill. — A fast, low-cost technique to see and count viruses or proteins from a sample in real time, without any chemicals or dyes, could underpin a new class of devices for rapid diagnostics and viral load monitoring, including HIV and the virus that causes COVID-19.
Researchers at the University of Illinois Urbana-Champaign described the technique, called Photonic Resonator Interferometric Scattering Microscopy, or PRISM, in the journal Nature Communications.
"We have developed a new form of microscopy that amplifies the interaction between light and biological materials. We can use it for very rapid and sensitive forms of diagnostic testing, and also as a very powerful tool for understanding biological processes at the scale of individual items, like counting individual proteins or recording individual protein interactions," said study leader Brian Cunningham, the Intel Alumni Endowed Chair of electrical and computer engineering and a member of the Holonyak Micro and Nanotechnology Lab and the Carl R. Woese Institute for Genomic Biology at Illinois.
In optical microscopes, light bounces off any molecules or viruses it encounters on a slide, creating a signal. Instead of a regular glass slide, the PRISM technique uses photonic crystal: a nanostructured glass surface that brilliantly reflects only one wavelength of light. Cunningham's group designed and fabricated a photonic crystal that reflects red light, so that the light from a red laser would be amplified.
"The molecules we are looking at – in this study, viruses and small proteins – are extremely small. They cannot scatter enough light to create a signal that can be detected by a conventional optical microscope," said graduate student Nantao Li, the first author of the paper. "The benefit of using the photonic crystal is that it amplifies the light's intensity so it's easier to detect those signals and enables us to study these proteins and viruses without any chemical labels or dyes that might modify their natural state or hinder their activity – we can just use the intrinsic scattering signal as the gauge for determining if those molecules are present."
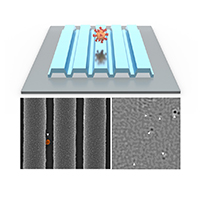
PRISM for COVID-19 detection. At top, concept art. Bottom left, a microscope image of a single virus on the photonic crystal surface. Bottom right, a PRISM image with six viruses detected.
Image courtesy of Nantao Li
The researchers verified their technique by detecting the virus that causes COVID-19. PRISM detected individual coronaviruses as they traveled across the slide's surface. The researchers also used PRISM to detect individual proteins such as ferritin and fibrinogen. The technique could allow researchers to study such biological targets in their natural states – watching as proteins interact, for example – or researchers could seed the surface of the photonic crystal slide with antibodies or other molecules to capture the targeted items and hold them in place.
"It takes 10 seconds to get a measurement, and in that time we can count the number of viruses captured on the sensor," Cunningham said. "It's a single-step detection method that works at room temperature. It is also fast, very sensitive and low cost. It's very different from the standard way we do viral testing now, which involves breaking open the viruses, extracting their genetic material and putting it through a chemical amplification process so we can detect it. That method, called PCR, is accurate and sensitive, but it requires time, specialized equipment and trained technicians."
Cunningham's group is working to incorporate PRISM technology into portable, rapid diagnostic devices for COVID-19 and HIV viral load monitoring. The group is exploring prototype devices that incorporate filters for blood samples and even condensation chambers for breath tests.
"We are also going to use this as a research tool for biology and cancer," Cunningham said. "We can use it to understand protein interactions that are parts of disease processes. We are interested in using it to detect these tiny vesicles that cancer cells shed, and to see what tissues they come from, for diagnosis, and also to study what cargo they are transporting from the cancer cells."
The National Science Foundation and the National Institutes of Health supported this work.






