Scientists have identified key molecules that mediate radioresistance in glioblastoma multiforme; these molecules are a potential target for the treatment of this brain cancer.
Glioblastoma multiforme (GBM), is the most aggressive type of brain cancer. It is treated by radiation therapy combined with chemotherapy. However, even with treatment, the five-year survival rate for GBM is less than 7%. One of the major causes for this is that GBM rapidly develops radioresistance (resistance to radiotherapy) by unknown mechanisms.
A team of scientists from Hokkaido University and Stanford University have revealed a mechanism by which GBM develops radioresistance. Their research, published in the journal Neuro-Oncology Advances, explains how two key molecules, Rab27b and epiregulin, interact to contribute to radioresistance in GBM.
The primary function of Rab27b is to regulate protein trafficking and secretion of molecules. Rab27b is also known to promote tumor progression and metastasis in several types of cancer. For these reasons, the scientists decided to investigate if Rab27b had any role to play in GBM.
Upon performing tests on human glioblastoma cell lines, the scientists showed that Rab27b expression was increased for at least seven days after exposure to radiation. Knockdown of Rab27b increased the sensitivity of glioblastoma cells to irradiation. These tests were replicated in an animal model: the glioblastoma cells were injected into mice, which were then subjected to radiation therapy. Rab27b knockdown combined with radiation therapy delayed tumor growth and prolonged mouse survival time.
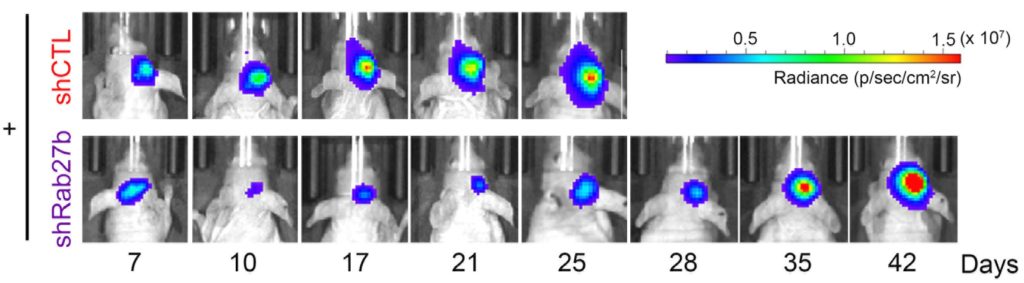
Tumor growth in mice injected with the glioblastoma cells. After radiotherapy, the growth of tumor with normal Rab27b expression (top row) is much more rapid than that of the tumor with knocked down Rab27b (bottom row). (Soichiro Nishioka, et al., Neuro-Oncology Advances, August 8, 2020).
As Rab27b is a regulator of protein trafficking, the scientists continued their work, looking for other molecules that contribute to radioresistance. They discovered that changes in the expression of Rab27b led to corresponding changes in the expression of epiregulin, a growth factor whose expression is known to increase in cancer cells; knocking down the expression of epiregulin increased the sensitivity to irradiation, as seen in the cells with Rab27b knockdown. Further, the scientists showed that increased expression of Rab27b and epiregulin in glioblastoma induced the proliferation of surrounding cancer cells, which could contribute to acquiring radioresistance. Finally, they analyzed gene expression data of GBM patients and found that upregulation of Rab27b and epiregulin correlated with poor prognosis of the patients.
By identifying the roles that Rab27b and epiregulin play in the development of radioresistance in GBM, the scientists have brought to light a novel target for drug development, and one that could significantly increase the survival rate for GBM.
Dr. Jin-Min Nam and Dr. Yasuhito Onodera are part of the Radiation Biology group at the Global Center for Biomedical Science and Engineering (GCB), a collaboration between Hokkaido University, Japan, and Stanford University, USA. The group specializes in molecular and cellular oncology, and radiation biology.

(Left to right) Yasuhito Ondera, Soichiro Nishioka, Jin-Min Nam and Ping-Hsui Wu from the Global Center for Biomedical Science and Engineering, Hokkaido University, who contributed to the current research (Photo: Jin-Min Nam).
Original Article:
Soichiro Nishioka, et al. Rab27b contributes to radioresistance and exerts a paracrine effect via epiregulin in glioblastoma. Neuro-Oncology Advances. August 8, 2020.
DOI: 10.1093/noajnl/vdaa091
Funding:
This study was supported by Grants-in-Aid from Ministry of Education, Science, Sports and Culture of Japan (MEXT; 19K08140 and 18H02759)