A research team led by Prof. NIE Guangjun from the National Center for Nanoscience and Technology (NCNST) of the Chinese Academy of Sciences (CAS) and collaborators have demonstrated a tumor membrane antigens-based nanovaccine derived from liposomal doxorubicin treated tumor tissues, which is efficacious in inducing a potent immunological defense against tumors. The study was published online in Cell Reports Medicine.
For solid tumor surgeries, challenges remain in postoperative tumor recurrence and metastasis. The correlation between postoperative tumor recurrence and metastasis and the host's antitumor immune status is well-established. Personalized cancer vaccines, using the patient's own tumor as an antigen source, stimulate a robust immune response that is efficacious in eliminating residual neoplastic foci following surgical intervention as well as in targeting metastatic lesions at a distance, significantly reducing the risk of postoperative tumor recurrence and metastasis.
The efficacy of autologous tumor cells in clinical trials has been limited by their weak immunogenicity. The tumor membrane contains tumor-presented antigens and associated antigens, which can be developed into a personalized antigen library that more accurately reflects the expression of tumor antigens. Vaccines based on autologous tumor cell membrane antigens have been developed. In clinical practice, patients administered autologous tumor cell membrane-based vaccines (TMVs) frequently have a history of prior therapeutic interventions with chemotherapy being the predominant modality. However, chemotherapy has the potential to alter the immunogenic properties of tumor cell membranes, consequently impacting the therapeutic efficacy of the TMVs.
In this study, researchers investigated the effects of preoperative chemotherapy on the efficacy of TMVs employing nano-formulated liposomal doxorubicin (NP-Dox) as a preoperative medication. For the formulation of the TMVs, they obtained the tumor membrane as the antigen, and resiquimod (R848), a potent dual Toll-like receptor 7/8 agonist with excellent efficacy, was encapsulated in Poly (lactide-co-glycolic acid) (PLGA) nanoparticles to act as the adjuvant of the vaccine. The TMVs formulated from liposomal doxorubicin-treated tumors induced superior dendritic cell maturation and T cell activation compared to doxorubicin, demonstrating better efficacy in preventing recurrence and metastasis in the postoperative murine model, as well as in extending postoperative survival.
Mechanistically, researchers demonstrated that NP-Dox, as a paradigmatic inducer of immunogenic cell death in tumors, upregulates the expression of immune-related molecules on the tumor cell membrane, enhancing the immunogenicity of tumor membrane antigens. Moreover, they showed that NP-Dox improves the immunological status of the tumor microenvironment, creating favorable conditions for subsequent nanovaccine immunization.
"In previous study, we developed an integrated vaccine formulation based on autologous tumor membrane antigens and bacterial cytoplasmic membrane adjuvants, which could achieve enhanced antitumor immune responses with a favorable biosafety profile," said Assoc. Prof. ZHAO Ruifang from NCNST and the first author of this study. "However, for its clinical applications, we found that patients had undergone various treatments before using autologous tumor membrane vaccines with chemotherapy being relatively common."
"This study provides valuable insights into the clinical application of TMVs, demonstrating its potential in solid tumor treatments. Concurrently, in this study, we employ nano-drugs both pre-and postoperatively, and showcase the superiority of nanotechnology in the integrated application of neoadjuvant chemotherapy and tumor immunotherapy," said Prof. NIE Guangjun from NCNST and another first author of this study.
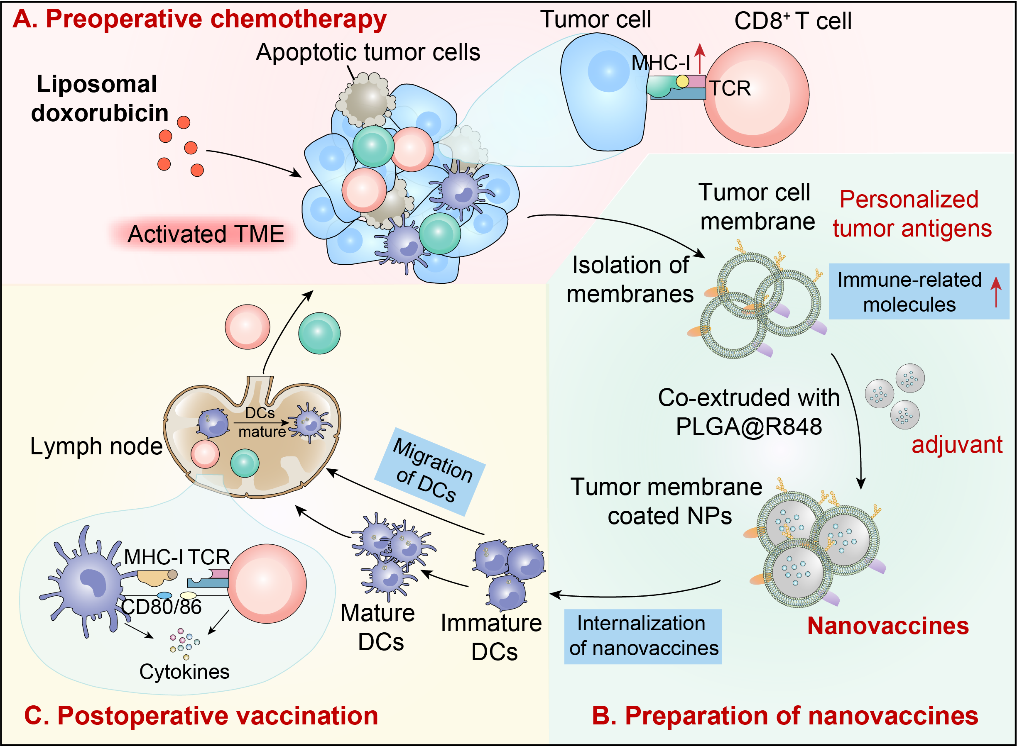
Schematic representation of nanotechnology's dual role in enhancing personalized tumor vaccine therapy in conjunction with neoadjuvant chemotherapy (Image by NIE Guangjun et al.)






