Delivering vaccines with this new technology will be cheaper and easier than liquid vaccines delivered by needles
The device—a Micro-projection Array Patch, or ‘MAP’—is a one square-centimeter of biomedical polymer – smaller than a postage stamp – embedded with 5,000 vaccine-coated micro-projections that pass through the skin’s outer layer to deliver a vaccine directly to thousands of immune-rich cells in the skin.
The result is more efficient vaccination that does not require refrigeration, like traditional vaccination with a needle and syringe.
The device is being commercialised by Australian biotech company Vaxxas, with manufacturing research being done by the University of Sydney and the Innovative Manufacturing Cooperative Research Centre (IMCRC).
"Delivering vaccines with this technology will be cheaper and easier than liquid vaccines delivered by needles because they don’t need to be refrigerated," said Cristyn Davies from the University of Sydney.
"This would offer a significant advantage in remote locations, including in developing countries where refrigeration to keep vaccines viable is a major challenge."
Delivering vaccines with this technology will be cheaper and easier than liquid vaccines delivered by needles.Cristyn Davies, University of Sydney
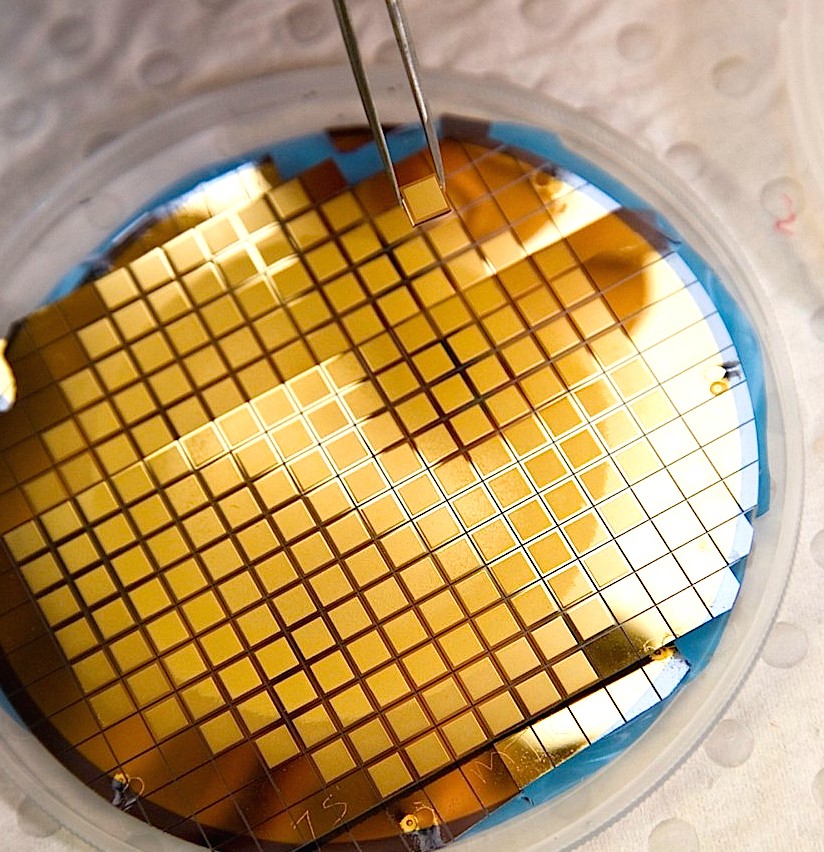
The device—a Micro-projection Array Patch, or ‘MAP’—is a one square-centimeter of biomedical polymer – smaller than a postage stamp
Cristyn Davies is evaluating the device with her University of Sydney colleagues Professor Rachel Skinner and Professor Robert Booy from Sydney Medical School and Professor Behnam Fahimnia from Sydney Business School.
Together, they will test the usability and acceptability of the device among patients and clinicians and assess its cost-effectiveness compared to conventional needles and cold-chain management.
Also, the device could boost vaccination rates since at least ten percent of people have been reported to avoid influenza vaccination due to fear of needles. Further, the World Health Organisation estimates there are 1.3 million deaths each year due to needlestick injuries and cross contamination.
Usability testing
The device is applied to the skin using a disposable applicator that contains the product and ensures reliable delivery.
In 2015 Vaxxas conducted a WHO-funded study testing the usability and acceptability of the applicator for polio vaccinations in Benin, Nepal and Vietnam.
Cristyn Davies said that study provided valuable information and suggested great potential for the Vaxxas product.
"With Vaxxas planning to develop and commercialise the device in Australia, our research will focus on how the applicator is perceived by patients and administrators," said Cristyn Davies.
Professor Rachel Skinner commented that Australia is a mature, developed vaccination market.
"What immunisation providers expect in using the device and its acceptance by immunisation providers and recipients may differ dramatically from those in developing countries", she said.
"We will be testing the application in several settings and across different age groups, in the workplace and with GPs," said Professor Booy.
The findings will be compared with results from the earlier WHO usability study to determine requirements in different markets.
"We are at an important stage of the product development process," said Charles Ross, Head of Clinical Operations at Vaxxas. "Before investing in manufacturing the applicator at pilot scale, we want to be confident the device satisfies design, end-user and logistical requirements for its intended markets."
"Vaxxas is a great example of manufacturing innovation in Australia," said David Chuter, CEO and Managing Director at IMCRC.
"This research will give Vaxxas end-user and distribution information that de-risks and accelerates the path to market and identifies product and design parameters required for subsequent pilot scale manufacture in Australia".
The Vaxxas MAP vaccine delivery system was invented at the University of Queensland and now is continuing development at the Translational Research Institute.
Fast facts
- The technology has the potential to completely disrupt the vaccine industry, a $30 billion market.
- Data from preclinical studies suggests the Vaxxas device will not only enhance the immune response generated by a vaccine compared to traditional delivery methods but potentially do this at a fraction of a full vaccine dose.
- Contrary to conventional vaccines, the vaccines tested by Vaxxas to date do not need to be refrigerated, reducing costs and alleviating transportation issues, particularly to parts of the world where cold chain infrastructure is unreliable.
- The Vaxxas device eliminates the risk of needle stick injuries. In addition, it will reduce the burden on patients suffering from needle-phobia.






