The findings could lead to a potential treatment for a common cause of blindness
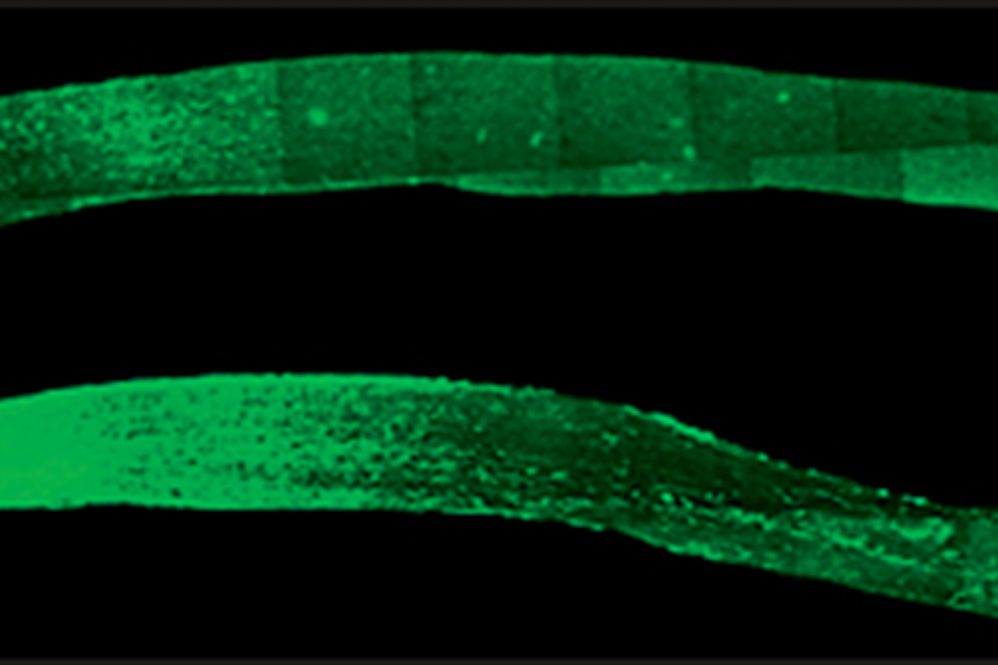
Image show two optic nerves (Photo courtesy of Trakhtenberg Lab)
Injured optic nerves can regrow towards the brain if treated properly, University of Connecticut scientists report in the September issue of Experimental Neurology. The findings in mice could lead to a potential treatment for a common cause of blindness.
Blunt trauma to the optic nerve from car crashes and accidents can lead to blindness. Glaucoma can cause similar damage to the optic nerve and is the second leading cause of blindness worldwide, according to the Centers for Disease Control. Loss of vision from blunt trauma and glaucoma is usually permanent, as severed nerves rarely grow back.
But recent findings by UConn School of Medicine neuroscientist Ephraim Trakhtenberg and members of his lab including Agnieszka Lukomska (now a professor at the University of Warsaw), Bruce Rheaume (currently a physician-resident at Dartmouth Medical Center), and Ph.D. candidate Matthew Frost have spread hope that the nerve damage can be reversed. Using an injectable peptide-a small piece of a larger protein-the team managed to get nerve cells in the severed optic nerves of mice to regrow from the damaged area all the way to the optic chiasm in the brain. The optic chiasm senses light and controls daily body rhythms. It is the first place in the brain the optic nerve touches.
It took the severed nerve cells about six weeks to regrow to the optic chiasm. It's unknown how much further they would have grown if the experiment had gone on longer.
"We need to do longer trials, at least three months, to reach further targets in the brain," Trakhtenberg says.
The idea to encourage nerve cell regrowth by injecting peptides came from a series of experiments exploring the ways nerve cells regrow, or don't, after trauma. It was well known that creating serious inflammation in the eye before nerve injury could induce some nerve cell regrowth afterward. But such a treatment is impractical for humans, both because it is impossible to anticipate a traumatic injury and because eye inflammation is generally undesirable.
Instead, Trakhtenberg's lab focuses on discovering what it is about inflammation that encourages nerves to regenerate. For example, one of the things that inflammation does is draw in macrophages. Macrophages are immune cells that do many things, including secrete a large protein called fibronectin.
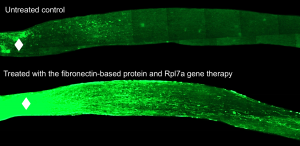
Over the course of many experiments, the researchers found that fibronectin seemed to encourage optic nerve cells to regrow. Nerve cells interacting with fibronectin tend to regrow. Nerve cells without fibronectin interactions don't.
But in most people with optic nerve injuries, nerve cells don't get a chance for much interaction with fibronectin due to its low levels around nerves. Most eye injuries don't cause enough inflammation to attract macrophages. And doctors cannot inject fibronectin into the eye. It's too large a protein to inject into the eye and have it diffuse into the optic nerve. But the Trakhtenberg lab team thought they might have another way.
They broke fibronectin up into pieces, short segments of protein called peptides. Peptides are much smaller than whole fibronectin, and easily injectable. They then took the peptide that interacted most strongly with optic nerve cells, synthesized it, and then injected it into the eyes of mice who had had their optic nerves injured.
The results were dramatic. Many more nerve cells survived in the mice that received the fibronectin peptide injection. And many of them began to regrow. In just 6 weeks, the optic nerves had dense regrowth through the spot of injury, and many nerve cells reached all the way to the optic chiasm in the brain. The best results were in mice that received gene therapy along with the fibronectin peptide injection, but the peptides alone also produced strong growth in the nerve fibers.
"One of the highlights is that the peptide is just a piece of protein. You can inject it. That gives it a therapeutic potential," for clinical trials as a treatment, Trakhtenberg says.
The team does not have a clinical trial lined up yet, but they hope to in the future. For now, they are focusing on running a longer trial in mice, at least three months, to see how far the regenerated nerve fibers actually grow and whether they eventually reach the area of the brain responsible for sight. They also want to experiment with combining other potential treatments with the peptides, to see if they can improve the nerve regrowth even more.






