An international team of scientists has unveiled new insights into the dissociation dynamics of sulfur hexafluoride (SF6) under high-energy X-ray excitation. The study, conducted using advanced synchrotron radiation techniques, sheds light on the formation of neutral sulfur atoms during the decay of deep core holes in SF6.
Understanding the interaction of X-rays with matter is fundamental to both scientific research and practical applications, including medical and technological advancements. These interactions involve complex processes such as absorption, ionization, scattering, and the decay of excited states, which emit electrons or photons.
In 1978, young scientists named Joseph Nordgren and Hans Ågren discovered an unusual spectral feature in sulfur hexafluoride (SF6) that defied explanation at the time. His discovery was made at the Siegbahn Laboratory of Uppsala University, founded by the late Nobel Prize laureate Kai Siegbahn. Despite further investigations, the nature of this spectral anomaly remained unclear.
Over 40 years later, Nordgren, now a prominent emeritus professor, revisited this mystery, developing a new hypothesis to interpret the feature. To uncover the hidden nature of the SF6 spectrum, the scientist collaborated with an international team from Europe and Japan, leveraging modern X-ray sources.
Using advanced synchrotron radiation techniques, the study provided insight into the formation of neutral sulfur atoms during the decay of deep core holes in SF6. In the experiment, SF6 molecules were exposed to hard X-rays that excited sulfur's innermost electron shells, initiating a cascade of electronic decays leading to the molecule's complete dissociation.
The team, under the experimental leadership of Nordgren, Oksana Travnikova from CNRS, and Florian Trinter from FHI, recorded soft X-ray emissions from intermediate states, a challenging endeavor due to the differing penetration depths of hard and soft X-rays in materials. Remarkably, the dissociation resulted in the formation of a neutral but excited sulfur atom, even though multiple electrons were ejected during the cascade.
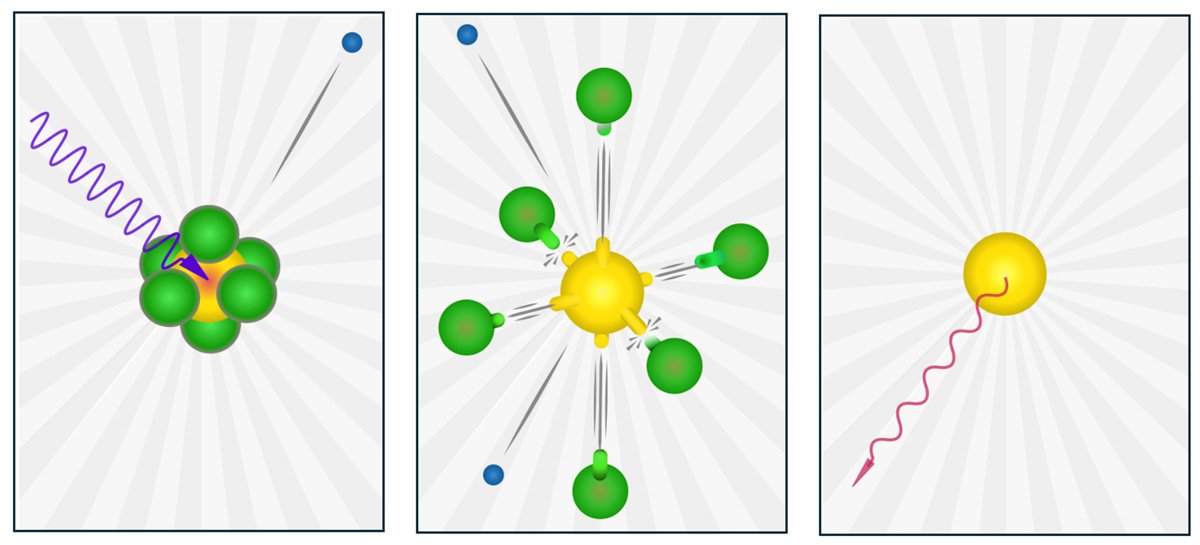
This phenomenon is at first surprising, given that fluorine, the most electronegative element in the periodic table, is known for its strong tendency to attract electron density. However, as the study reveals, the fluorine atoms in this case instead counterbalance for the electron density loss in the sulfur atom during the intermediate states.
In the final step of the cascade, the system emits soft X-rays of a specific energy that cannot be associated with molecular fragments (such as sulfur bound to fluorine) or any charged sulfur state. Advanced ab initio theoretical calculations were critical for interpreting the observed soft X-ray emissions and for understanding the complex molecular response to deep inner-shell ionization of sulfur in SF6. This study demonstrates the power of modern X-ray techniques in unraveling long-standing scientific mysteries and advancing our understanding of molecular dynamics.
The journey of discovery spans decades, showing that persistence and patience are sometimes as crucial as innovation.
- Publication Details:
Title: Neutral Sulfur Atom Formation in Decay of Deep Core Holes in SF6
Authors: Oksana Travnikova, Florian Trinter, Marcus Agåker, Giorgio Visentin, Joakim Andersson, Ludvig Kjellsson, Iyas Ismail, Nicolas Velasquez, Dimitris Koulentianos, Zhong Yin, Johan Söderström, Tatiana Marchenko, Renaud Guillemin, O. Dennis McGinnis, Hans Ågren, Stephan Fritzsche, Marc Simon, Jan-Erik Rubensson, Joseph Nordgren
Journal: Physical Review Letters






